- Cover
- Title Page
- Dedication
- Epigraph
- Contents
- The Basics of Human Brain Anatomy
- Our Evolutionary Lineage
- Introduction
- 1: The World Before Brains
- Breakthrough #1: Steering and the First Bilaterians
- Breakthrough #2: Reinforcing and the First Vertebrates
- Breakthrough #3: Simulating and the First Mammals
- Breakthrough #4: Mentalizing and the First Primates
- Breakthrough #5: Speaking and the First Humans
- Conclusion: The Sixth Breakthrough
- Acknowledgments
- Glossary
- Bibliography
- Notes
- Index
- About the Author
- Praise for A Brief History of Intelligence
- Copyright
- About the Publisher
Dedication
To my wife, Sydney
Epigraph
In the distant future I see open fields for far more important researches. Psychology will be based on a new foundation, that of the necessary acquirement of each mental power and capacity by gradation. Light will be thrown on the origin of man and his history.
—CHARLES DARWIN IN 1859
Contents
The Basics of Human Brain Anatomy
Breakthrough #1: Steering and the First Bilaterians
4: Associating, Predicting, and the Dawn of Learning
Breakthrough #2: Reinforcing and the First Vertebrates
6: The Evolution of Temporal Difference Learning
7: The Problems of Pattern Recognition
9: The First Model of the World
Breakthrough #3: Simulating and the First Mammals
11: Generative Models and the Neocortical Mystery
13: Model-Based Reinforcement Learning
14: The Secret to Dishwashing Robots
Breakthrough #4: Mentalizing and the First Primates
15: The Arms Race for Political Savvy
17: Monkey Hammers and Self-Driving Cars
18: Why Rats Can’t Go Grocery Shopping
Breakthrough #5: Speaking and the First Humans
19: The Search for Human Uniqueness
22: ChatGPT and the Window into the Mind
Conclusion: The Sixth Breakthrough
The Basics of Human Brain Anatomy
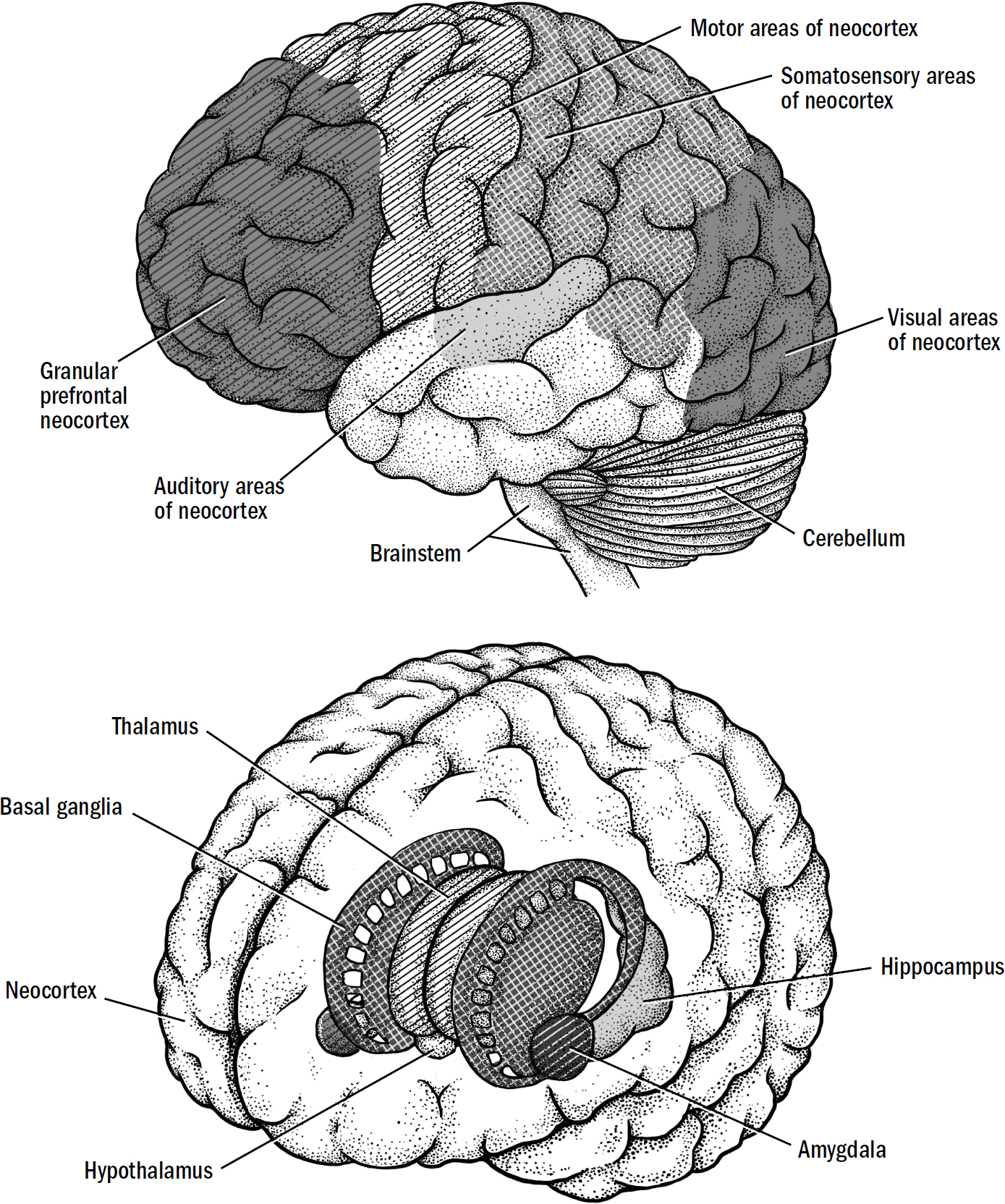
Original art by Mesa Schumacher
Our Evolutionary Lineage
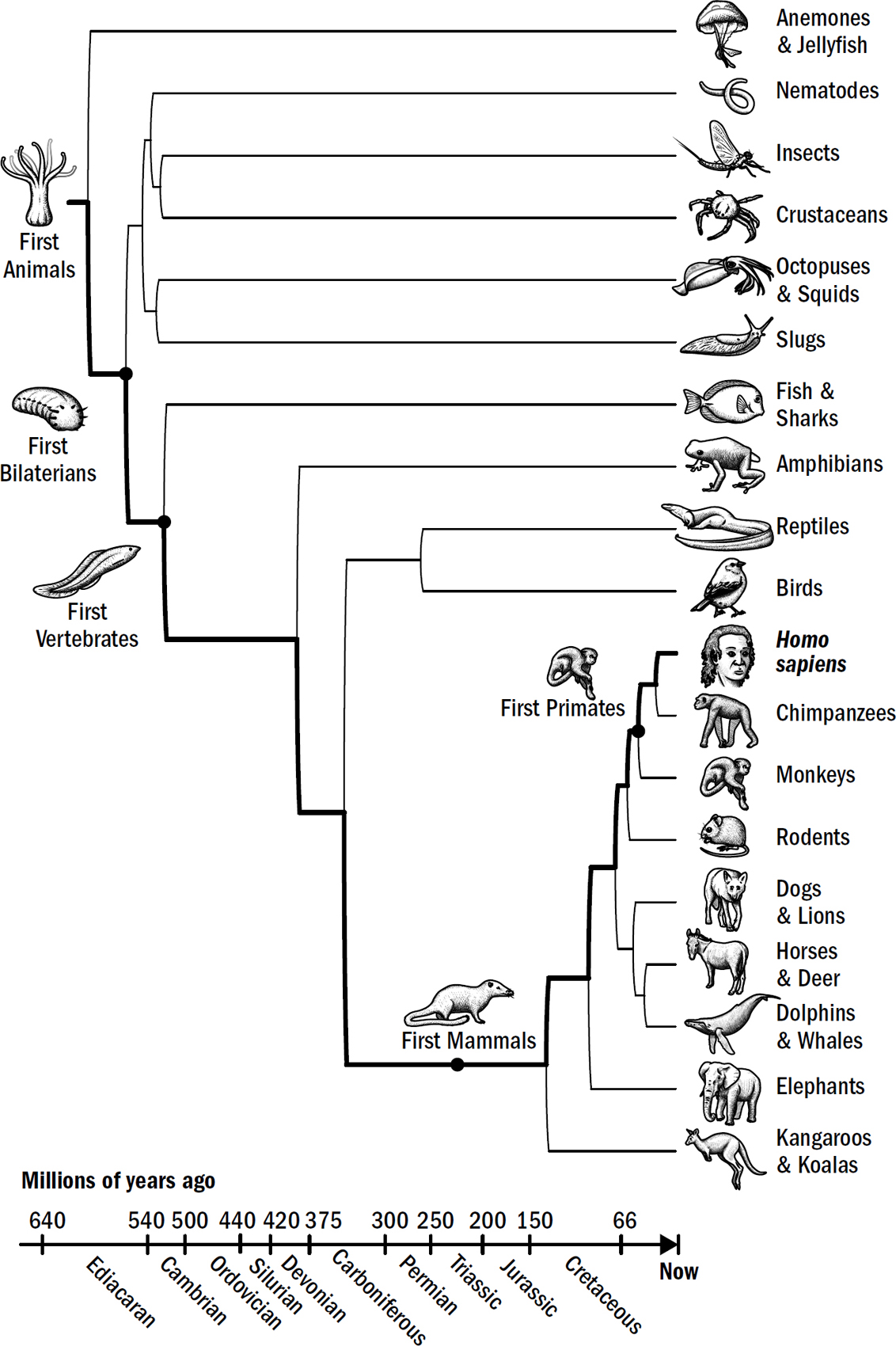
Original art by Rebecca Gelernter
Special thanks to Rebecca Gelernter for creating the incredible original art in this book; Rebecca created the art at the beginning of each Breakthrough section and designed the majority of the figures. Also, a special thanks to Mesa Schumacher for her wonderful original anatomical art of the human, lamprey, monkey, and rat brain that she made specifically for this book.
Introduction
IN SEPTEMBER 1962, during the global tumult of the space race, the Cuban missile crisis, and the recently upgraded polio vaccine, there was a less reported—but perhaps equally critical—milestone in human history: It was in the fall of ’62 that we predicted the future.
Cast onto the newly colorful screens of American televisions was the debut of The Jetsons, a cartoon about a family living one hundred years in the future. In the guise of a sitcom, the show was, in fact, a prediction of how future humans would live, of what technologies would fill their pockets and furnish their homes.
The Jetsons correctly predicted video calls, flat-screen TVs, cell phones, 3D printing, and smartwatches; all technologies that were unbelievable in 1962 and yet were ubiquitous by 2022. However, there is one technology that we have entirely failed to create, one futurist feat that has not yet come to fruition: the autonomous robot named Rosey.
Rosey was a caretaker for the Jetson family, watching after the children and tending to the home. When Elroy—then six years old—was struggling in school, it was Rosey who helped him with his homework. When their fifteen-year-old daughter, Judy, needed help learning how to drive, it was Rosey who gave her lessons. Rosey cooked meals, set the table, and did the dishes. Rosey was loyal, sensitive, and quick with a joke. She identified brewing family tiffs and misunderstandings, intervening to help individuals see one another’s perspective. At one time, she was moved to tears by a poem Elroy wrote for his mother. Rosey herself, in one episode, even fell in love.
In other words, Rosey had the intelligence of a human. Not just the reasoning, common sense, and motor skills needed to perform complex tasks in the physical world, but also the empathizing, perspective taking, and social finesse needed to successfully navigate our social world. In the words of Jane Jetson, Rosey was
Although the The Jetsons correctly predicted cell phones and smartwatches, we still don’t have anything like Rosey. As of this book going to print, even Rosey’s most basic behaviors are still out of reach. It is no secret that the first company to build a robot that can simply load a dishwasher will immediately have a bestselling product. All attempts to do this have failed. It isn’t fundamentally a mechanical problem; it’s an intellectual one—the ability to identify objects in a sink, pick them up appropriately, and load them without breaking anything has proven far more difficult than previously thought.
Of course, even though we do not yet have Rosey, the progress in the field of artificial intelligence (AI) since 1962 has been remarkable. AI can now beat the best humans in the world at numerous games of skill, including chess and Go. AI can recognize tumors in radiology images as well as human radiologists. AI is on the cusp of autonomously driving cars. And as of the last few years, new advancements in large language models are enabling products like ChatGPT, which launched in fall 2022, to compose poetry, translate between languages at will, and even write code. To the chagrin of every high school teacher on planet Earth, ChatGPT can instantly compose a remarkably well written and original essay on almost any topic that an intrepid student might ask of it. ChatGPT can even pass the bar exam, scoring better than 90 percent of lawyers.
Along this journey, as AI keeps getting smarter, it is becoming harder to measure our progress toward this goal. If an AI system outperforms humans on a task, does it mean that the AI system has captured how humans solve the task? Does a calculator—capable of crunching numbers faster than a human—actually understand math? Does ChatGPT—scoring better on the bar exam than most lawyers—actually understand the law? How can we tell the difference, and in what circumstances, if any, does the difference even matter?
In 2021, over a year before the release of ChatGPT—the chatbot that is now rapidly proliferating throughout every nook and cranny of society—I was using its precursor, a large language model called GPT-3. GPT-3 was trained on large quantities of text (large as in the entire internet), and then used this corpus to try to pattern match the most likely response to a prompt. When asked, “What are two reasons that a dog might be in a bad mood?” it responded, “Two reasons a dog might be in a bad mood are if it is hungry or if it is hot.” Something about the new architecture of these systems enabled them to answer questions with what at least seemed like a remarkable degree of intelligence. These models were able to generalize facts they had read about (like the Wikipedia pages about dogs and other pages about causes of bad moods) to new questions they had never seen. In 2021, I was exploring possible applications of these new language models—could they be used to provide new support systems for mental health, or more seamless customer service, or more democratized access to medical information?
Indeed, the discrepancies between artificial intelligence and human intelligence are nothing short of perplexing. Why is it that AI can crush any human on earth in a game of chess but can’t load a dishwasher better than a six-year-old?
We struggle to answer these questions because we don’t yet understand the thing we are trying to re-create. All of these questions are, in essence, not questions about AI, but about the nature of human intelligence itself—how it works, why it works the way it does, and as we will soon see, most importantly, how it came to be.
Nature’s Hint
When humanity wanted to understand flight, we garnered our first inspiration from birds; when George de Mestral invented Velcro, he got the idea from burdock fruits; when Benjamin Franklin sought to explore electricity, his first sparks of understanding came from lightning. Nature has, throughout the history of human innovation, long been a wondrous guide.
Nature also offers us clues as to how intelligence works—the clearest locus of which is, of course, the human brain. But in this way, AI is unlike these other technological innovations; the brain has proven to be more unwieldy and harder to decipher than either wings or lightning. Scientists have been investigating how the brain works for millennia, and while we have made progress, we do not yet have satisfying answers.
The problem is complexity.
The human brain contains eighty-six billion neurons and over a hundred trillion connections. Each of those connections is so minuscule—less than thirty nanometers wide—that they can barely be seen under even the most powerful microscopes. These connections are bunched together in a tangled mess—within a single cubic millimeter (the width of a single letter on a penny), there are
But the sheer number of connections is only one aspect of what makes the brain complex; even if we mapped the wiring of each neuron we would still be far from understanding how the brain works. Unlike the electrical connections in your computer, where wires all communicate using the same signal—electrons—across each of these neural connections, hundreds of different chemicals are passed, each with completely different effects. The simple fact that two neurons connect to each other tells us little about what they are communicating. And worst of all, these connections themselves are in a constant state of change, with some neurons branching out and forming new connections, while others are retracting and removing old ones. Altogether, this makes reverse engineering how the brain works an ungodly task.
Studying the brain is both tantalizing and infuriating. One inch behind your eyes is the most awe-inspiring marvel of the universe. It houses the secrets to the nature of intelligence, to building humanlike artificial intelligence, to why we humans think and behave the way we do. It is right there, reconstructed millions of times per year with every newly born human. We can touch it, hold it, dissect it, we are literally made of it, and yet its secrets remain out of reach, hidden in plain sight.
If we want to reverse-engineer how the brain works, if we want to build Rosey, if we want to uncover the hidden nature of human intelligence, perhaps the human brain is not nature’s best clue. While the most intuitive place to look to understand the human brain is, naturally, inside the human brain itself, counterintuitively, this may be the last place to look. The best place to start may be in dusty fossils deep in the Earth’s crust, in microscopic genes tucked away inside cells throughout the animal kingdom, and in the brains of the many other animals that populate our planet.
In other words, the answer might not be in the present, but in the hidden remnants of a long time past.
The Missing Museum of Brains
—GEOFFREY HINTON (PROFESSOR AT UNIVERSITY OF TORONTO, CONSIDERED ONE OF THE “GODFATHERS OF AI”)
Humans fly spaceships, split atoms, and edit genes. No other animal has even invented the wheel.
Because of humanity’s larger résumé of inventions, you might think that we would have little to learn from the brains of other animals. You might think that the human brain would be entirely unique and nothing like the brains of other animals, that some special brain structure would be the secret to our cleverness. But this is not what we see.
What is most striking when we examine the brains of other animals is how remarkably similar their brains are to our own. The difference between our brain and a chimpanzee’s brain, besides size, is barely anything. The difference between our brain and a rat’s brain is only a handful of brain modifications. The brain of a fish has almost all the same structures as our brain.
These similarities in brains across the animal kingdom mean something important. They are clues. Clues about the nature of intelligence. Clues about ourselves. Clues about our past.
Although today brains are complex, they were not always so. The brain emerged from the unthinking chaotic process of evolution; small random variations in traits were selected for or pruned away depending on whether they supported the further reproduction of the life-form.
If only we could go back in time and examine this first brain to understand how it worked and what tricks it enabled. If only we could then track the complexification forward in the lineage that led to the human brain, observing each physical modification that occurred and the intellectual abilities it afforded. If we could do this, we might be able to grasp the complexity that eventually emerged. Indeed, as the biologist Theodosius Dobzhansky famously said, “Nothing in biology makes sense except in the light of evolution.”
Even Darwin fantasized about reconstructing such a story. He ends his Origin of Species fantasizing about a future when “psychology will be based on a new foundation, that of the necessary acquirement of each mental power and capacity by gradation.” One hundred fifty years after Darwin, this may finally be possible.
Although we have no time machines, we can, in principle, engage in time travel. In just the past decade, evolutionary neuroscientists have made incredible progress in reconstructing the brains of our ancestors. One way they do this is through the fossil record—scientists can use the fossilized skulls of ancient creatures to reverse-engineer the structure of their brains. Another way to reconstruct the brains of our ancestors is by examining the brains of other animals in the animal kingdom.
The reason why brains across the animal kingdom are so similar is that they all derive from common roots in shared ancestors. Every brain in the animal kingdom is a little clue as to what the brains of our ancestors looked like; each brain is not only a machine but a time capsule filled with hidden hints of the trillions of minds that came before. And by examining the intellectual feats these other animals share and those they do not, we can begin to not only reconstruct the brains of our ancestors, but also determine what intellectual abilities these ancient brains afforded them. Together, we can begin to trace acquirement of each mental power by gradation.
It is all, of course, still a work in progress, but the story is becoming tantalizingly clear.
The Myth of Layers
I am hardly the first to propose an evolutionary framework for understanding the human brain. There is a long tradition of such frameworks. The most famous was formulated in the 1960s by the neuroscientist Paul MacLean. MacLean hypothesized that the human brain was made of three layers (hence triune), each built on top of another: the neocortex, which evolved most recently, on top of the limbic system, which evolved earlier, on top of the reptile brain, which evolved first.
MacLean argued that the reptile brain was the center of our basic survival instincts, such as aggression and territoriality. The limbic system was supposedly the center of emotions, such as fear, parental attachment, sexual desire, and hunger. And the neocortex was supposedly the center of cognition, gifting us with language, abstraction, planning, and perception. MacLean’s framework suggested that reptiles had only a reptile brain, mammals like rats and rabbits had a reptile brain and a limbic system, and we humans had all three systems. Indeed, to him, these “three evolutionary formations might be imagined as three interconnected biological computers, with each having its own special intelligence, its own subjectivity, its own sense of time and space, and its own memory,
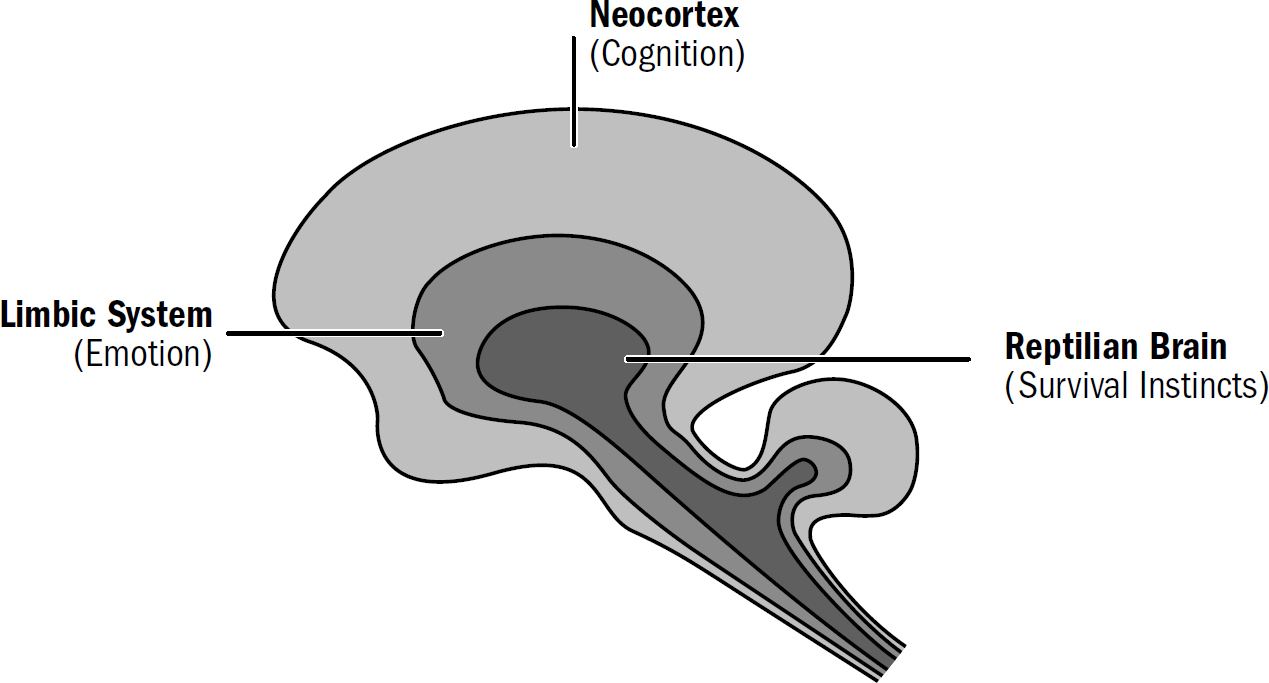
Figure 1: MacLean’s triune brain
Figure by Max Bennett (inspired by similar figures found in MacLean’s work)
But even if MacLean’s triune brain had turned out to be closer to the truth, its biggest problem is that its functional divisions aren’t particularly useful for our purposes. If our goal is to reverse-engineer the human brain to understand the nature of intelligence, MacLean’s three systems are too broad and the functions attributed to them too vague to provide us with even a point at which to start.
We need to ground our understanding of how the brain works and how it evolved in our understanding of how intelligence works—for which we must look to the field of artificial intelligence. The relationship between AI and the brain goes both ways; while the brain can surely teach us much about how to create artificial humanlike intelligence, AI can also teach us about the brain. If we think some part of the brain uses some specific algorithm but that algorithm doesn’t work when we implement it in machines, this gives us evidence that the brain might not work this way. Conversely, if we find an algorithm that works well in AI systems, and we find parallels between the properties of these algorithms and properties of animal brains, this gives us some evidence that the brain might indeed work this way.
The physicist Richard Feynman left the following on a blackboard shortly before his death: “What I cannot create, I do not understand.” The brain is our guiding inspiration for how to build AI, and AI is our litmus test for how well we understand the brain.
We need a new evolutionary story of the brain, one grounded not only in a modern understanding of how brain anatomy changed over time, but also in a modern understanding of intelligence itself.
The Five Breakthroughs
—YANN LECUN, HEAD OF AI AT META
We have a lot of evolutionary history to cover—four billion years. Instead of chronicling each minor adjustment, we will be chronicling the major evolutionary breakthroughs. In fact, as an initial approximation—a first template of this story—the entirety of the human brain’s evolution can be reasonably summarized as the culmination of only five breakthroughs, starting from the very first brains and going all the way to human brains.
These five breakthroughs are the organizing map to our book, and they make up our itinerary for our adventure back in time. Each breakthrough emerged from new sets of brain modifications and equipped animals with a new portfolio of intellectual abilities. This book is divided into five parts, one for each breakthrough. In each section, I will describe why these abilities evolved, how they worked, and how they still manifest in human brains today.
Each subsequent breakthrough was built on the foundation of those that came before and provided the foundation for those that would follow. Past innovations enabled future innovations. It is through this ordered set of modifications that the evolutionary story of the brain helps us make sense of the complexity that eventually emerged.
But this story cannot be faithfully retold by considering only the biology of our ancestors’ brains. These breakthroughs always emerged from periods when our ancestors faced extreme situations or got caught in powerful feedback loops. It was these pressures that led to rapid reconfigurations of brains. We cannot understand the breakthroughs in brain evolution without also understanding the trials and triumphs of our ancestors: the predators they outwitted, the environmental calamities they endured, and the desperate niches they turned to for survival.
And crucially, we will ground these breakthroughs in what is currently known in the field of AI, for many of these breakthroughs in biological intelligence have parallels to what we have learned in artificial intelligence. Some of these breakthroughs represent intellectual tricks we understand well in AI, while other tricks still lay beyond our understanding. And in this way, perhaps the evolutionary story of the brain can shed light on what breakthroughs we may have missed in the development of artificial humanlike intelligence. Perhaps it will reveal some of nature’s hidden clues.
Me
I wish I could tell you that I wrote this book because I have spent my whole life pondering the evolution of the brain and trying to build intelligent robots. But I am not a neuroscientist or a roboticist or even a scientist. I wrote this book because I wanted to read this book.
I came to the perplexing discrepancy between human and artificial intelligence by trying to apply AI systems to real-world problems. I spent the bulk of my career at a company I cofounded named Bluecore; we built software and AI systems to help some of the largest brands in the world personalize their marketing. Our software helped predict what consumers would buy before they knew what they wanted. We were merely one tiny part in a sea of countless companies beginning to use the new advances in AI systems. But all these many projects, both big and small, were shaped by the same perplexing questions.
When commercializing AI systems, there is eventually a series of meetings between business teams and machine learning teams. The business teams look for applications of new AI systems that would be valuable, while only the machine learning teams understand what applications would be feasible. These meetings often reveal our mistaken intuitions about how much we understand about intelligence. Businesspeople probe for applications of AI systems that seem straightforward to them. But frequently, these tasks seem straightforward only because they are straightforward for our brains. Machine learning people then patiently explain to the business team why the idea that seems simple is, in fact, astronomically difficult. And these debates go back and forth with every new project. It was from these explorations into how far we could stretch modern AI systems and the surprising places where they fall short that I developed my original curiosity about the brain.
Of course, I am also a human and I, like you, have a human brain. So it was easy for me to become fascinated with the organ that defines so much of the human experience. The brain offers answers not only about the nature of intelligence, but also why we behave the way we do. Why do we frequently make irrational and self-defeating choices? Why does our species have such a long recurring history of both inspiring selflessness and unfathomable cruelty?
My personal project began with merely trying to read books to answer my own questions. This eventually escalated to lengthy email correspondences with neuroscientists who were generous enough to indulge the curiosities of an outsider. This research and these correspondences eventually led me to publish several research papers, which all culminated in the decision to take time off work to turn these brewing ideas into a book.
Throughout this process, the deeper I went, the more I became convinced that there was a worthwhile synthesis to be contributed, one that could provide an accessible introduction to how the brain works, why it works the way it does, and how it overlaps and differs from modern AI systems; one that could bring various ideas across neuroscience and AI together under an umbrella of a single story.
A Brief History of Intelligence is a synthesis of the work of many others. At its heart, it is merely an attempt to put together the pieces that were already there. I have done my best to give due credit throughout the book, always aiming to celebrate those scientists who did the actual research. Anywhere I have failed to do so is unintentional. Admittedly, I couldn’t resist sprinkling in a few speculations of my own, but I will aim to be clear when I step into such territory.
It is perhaps fitting that the origin of this book, like the origin of the brain itself, came not from prior planning but from a chaotic process of false starts and wrong turns, from chance, iteration, and lucky circumstance.
A Final Point (About Ladders and Chauvinism)
I have one final point to make before we begin our journey back in time. There is a misinterpretation that will loom dangerously between the lines of this entire story.
This book will draw many comparisons between the abilities of humans and those of other animals alive today, but this is always done by picking specifically those animals that are believed to be most similar to our ancestors. This entire book—the five-breakthroughs framework itself—is solely the story of the human lineage, the story of how our brains came to be; one could just as easily construct a story of how the octopus or honeybee brain came to be, and it would have its own twists and turns and its own breakthroughs.
Just because our brains wield more intellectual abilities than those of our ancestors does not mean that the modern human brain is strictly intellectually superior to those of other modern animals.
Evolution independently converges on common solutions all the time. The innovation of wings independently evolved in insects, bats, and birds; the common ancestor of these creatures did not have wings. Eyes are also believed to have independently evolved many times. Thus, when I argue that an intellectual ability, such as episodic memory, evolved in early mammals, this does not mean that today only mammals have episodic memory. Like with wings and eyes, other lineages of life may have independently evolved episodic memory. Indeed, many of the intellectual faculties that we will chronicle in this book are not unique to our lineage, but have independently sprouted along numerous branches of earth’s evolutionary tree.
Since the days of Aristotle, scientists and philosophers have constructed what modern biologists refer to as a “scale of nature” (or, since scientists like using Latin terms, scala naturae). Aristotle created a hierarchy of all life-forms with humans being superior to other mammals, who were in turn superior to reptiles and fish, who were in turn superior to insects, who were in turn superior to plants.
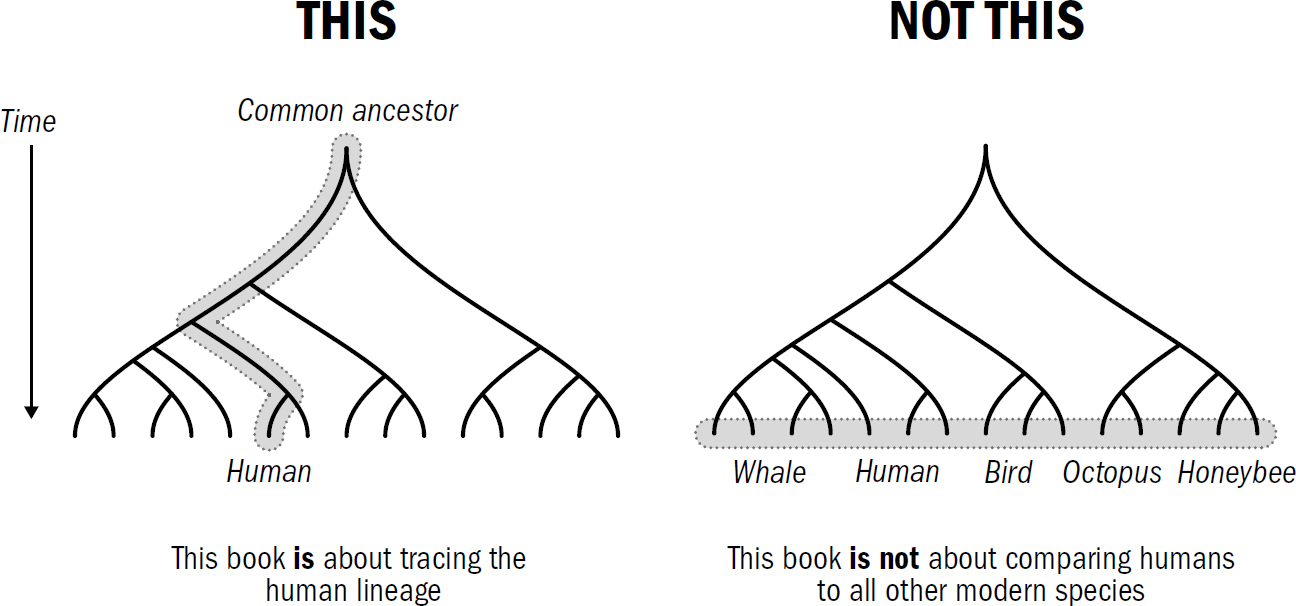
Figure 2
Original art by Rebecca Gelernter
Even after the discovery of evolution, the idea of a scale of nature continues to persist. This idea that there is a hierarchy of species is dead wrong. All species alive today are, well, alive; their ancestors survived the last 3.5 billion years of evolution. And thus, in that sense—the only sense that evolution cares about—all life-forms alive today are tied for first place.
Species fall into different survival niches, each of which optimizes for different things. Many niches—in fact, most niches—are better served by smaller and simpler brains (or no brains at all). Big-brained apes are the result of a different survival strategy than that of worms, bacteria, or butterflies. But none are “better.” In the eyes of evolution, the hierarchy has only two rungs: on one, there are those that survived, and on the other, those that did not.
My appeal: As we trace our story, we must avoid thinking that the complexification from past to future suggests that modern humans are strictly superior to modern animals. We must avoid the accidental construction of a scala naturae. All animals alive today have been undergoing evolution for the same amount of time.
However, there are, of course, things that make us humans unique, and because we are human, it makes sense that we hold a special interest in understanding ourselves, and it makes sense that we strive to make artificial humanlike intelligences. So I hope we can engage in a human-centered story without devolving into human chauvinism. There is an equally valid story to be told for any other animal, from honeybees to parrots to octopuses, with which we share our planet. But we will not tell these stories here. This book tells the story of only one of these intelligences: it tells the story of us.
1
The World Before Brains
LIFE EXISTED ON Earth for a long time—and I mean a long time, over three billion years—before the first brain made an appearance. By the time the first brains evolved, life had already persevered through countless evolutionary cycles of challenge and change. In the grand arc of life on Earth, the story of brains would not be found in the main chapters but in the epilogue—brains appeared only in the most recent 15 percent of life’s story. Intelligence too existed for a long time before brains; as we will see, life began exhibiting intelligent behavior early in its story. We cannot understand why and how brains evolved without first reviewing the evolution of intelligence itself.
Around four billion years ago, deep in the volcanic oceans of a lifeless Earth, just the right soup of molecules were bouncing around the microscopic nooks and crannies of
Although these self-replicating DNA-like molecules also succumbed to the destructive effects of entropy, they didn’t have to survive individually to survive collectively—as long as they endured long enough to create their own copies, they would, in essence, persist. This is the genius of self-replication. With these first self-replicating molecules, a primitive version of the process of evolution began; any new lucky circumstances that facilitated more successful duplication would, of course, lead to more duplicates.
There were two subsequent evolutionary transformations that led to life. The first was when protective lipid bubbles entrapped these DNA molecules using the same mechanism by which soap, also made of lipids, naturally bubbles when you wash your hands. These DNA-filled microscopic lipid bubbles were the first versions of cells, the fundamental unit of life.
The second evolutionary transformation occurred when a suite of nucleotide-based molecules—ribosomes—began translating specific sequences of DNA into specific sequences of amino acids that were then folded into specific three-dimensional structures we call proteins. Once produced, these proteins float around inside a cell or are embedded in the wall of the cell fulfilling different functions. You have probably, at least in passing, heard that your DNA is made up of genes. Well, a gene is simply the section of DNA that codes for the construction of a specific and singular protein. This was the invention of protein synthesis, and it is here that the first sparks of intelligence made their appearance.
DNA is relatively inert, effective for self-duplication but otherwise limited in its ability to manipulate the microscopic world around it. Proteins, however, are far more flexible and powerful. In many ways, proteins are more machine than molecule. Proteins can be constructed and folded into many shapes—sporting tunnels, latches, and other robotic moving parts—and can thereby subserve endless cellular functions, including “intelligence.”
Armed with proteins for movement and perception, early life could monitor and respond to the outside world. Bacteria can swim away from environments that lower the probability of successful replication, environments that have, for example, temperatures that are too hot or cold or chemicals that are destructive to DNA or cell membranes. Bacteria can also swim toward environments that are amenable to reproduction.
And in this way, these ancient cells indeed had a primitive version of intelligence, implemented not in neurons but in a complex network of chemical cascades and proteins.
The development of protein synthesis not only begot the seeds of intelligence but also transformed DNA from mere matter to a medium for storing information. Instead of being the self-replicating stuff of life itself, DNA was transformed into the informational foundation from which the stuff of life is constructed. DNA had officially become life’s blueprint, ribosomes its factory, and proteins its product.
With these foundations in place, the process of evolution was initiated in full force: variations in DNA led to variations in proteins, which led to the evolutionary exploration of new cellular machinery, which, through natural selection, were pruned and selected for based on whether they further supported survival. By this point in life’s story, we have concluded the long, yet-to-be-replicated, and mysterious process scientists call abiogenesis: the process by which nonbiological matter (abio) is converted into life (genesis).
The Terraforming of Earth
LUCA, living around 3.5 billion years ago, likely resembled a simpler version of a modern bacteria. And indeed, for a long time after this, all life was bacterial. After a further billion years—through trillions upon trillions of evolutionary iterations—Earth’s oceans were brimming with many diverse species of these microbes, each with its own portfolio of DNA and proteins. One way in which these early microbes differed from one another was in their systems of energy production. The story of life, at its core, is as much about energy as it is about entropy.
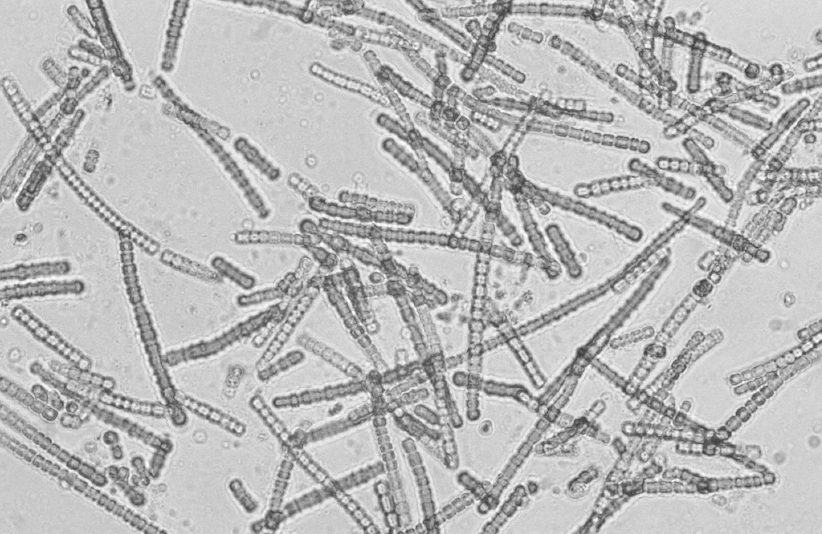
Photograph by Willem van Aken on March 18, 1993. Figure from www.scienceimage.csiro.au/image/4203 CC BY 3.0 license.
Life on Earth fell into perhaps the greatest symbiosis ever found between two competing but complementary systems of life, one that lasts to this day. One category of life was photosynthetic, converting water and carbon dioxide into sugar and oxygen. The other was respiratory, converting sugar and oxygen back into carbon dioxide. At the time, these two forms of life were similar, both single-celled bacteria. Today this symbiosis is made up of very different forms of life. Trees, grass, and other plants are some of our modern photosynthesizers, while fungi and animals are some of our modern respirators.
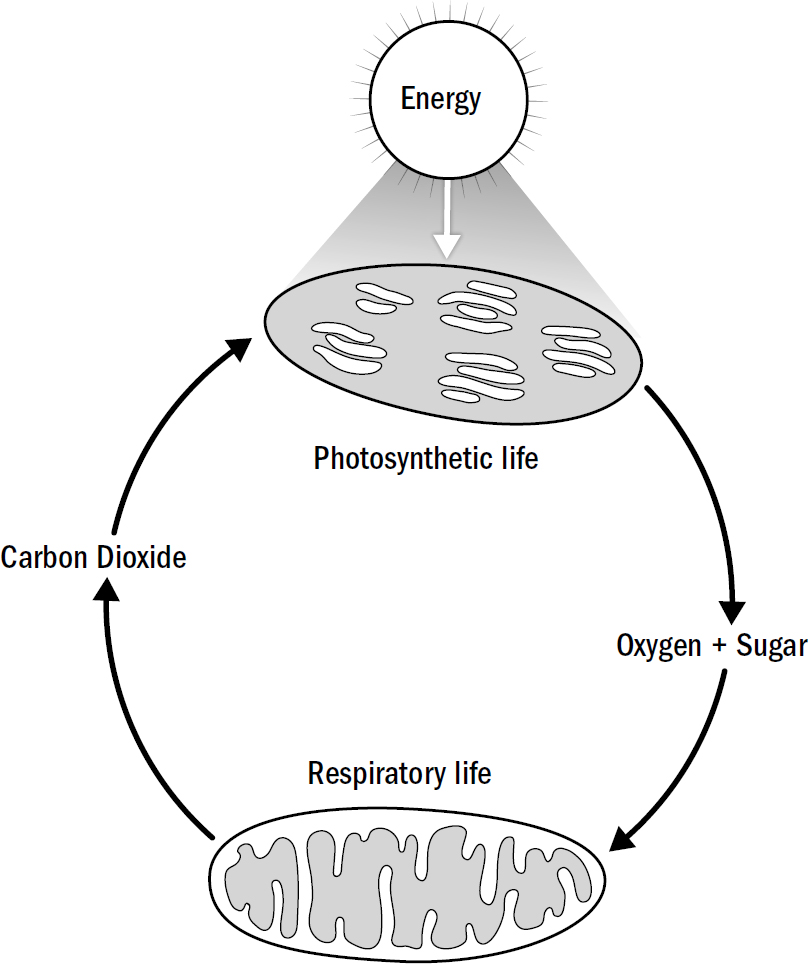
Figure 1.2: The symbiosis between photosynthetic and respiratory life
Original art by Rebecca Gelernter
Cellular respiration requires sugar to produce energy, and this basic need provided the energetic foundation for the eventual intelligence explosion that occurred uniquely within the descendants of respiratory life. While most, if not all, microbes at the time exhibited primitive levels of intelligence, it was only in respiratory life that intelligence was later elaborated and extended. Respiratory microbes differed in one crucial way from their photosynthetic cousins: they needed to hunt. And hunting required a whole new degree of smarts.
Three Levels
However, unlike the cells that came before, respiratory life could survive only by stealing the energetic prize—the sugary innards—of photosynthetic life. Thus, the world’s utopic peace ended quite abruptly with the arrival of aerobic respiration. It was here that microbes began to actively eat other microbes. This fueled the engine of evolutionary progress; for every defensive innovation prey evolved to stave off being killed, predators evolved an offensive innovation to overcome that same defense. Life became caught in an arms race, a perpetual feedback loop: offensive innovations led to defensive innovations that required further offensive innovations.
Original art by Rebecca Gelernter
What was common across these eukaryote lineages was that all three—plants, fungi, and animals—each independently evolved multicellularity. Most of what you see and think of as life—humans, trees, mushrooms—are primarily multicellular organisms, cacophonies of billions of individual cells all working together to create a singular emergent organism. A human is made up of exactly such diverse types of specialized cells: skin cells, muscle cells, liver cells, bone cells, immune cells, blood cells. A plant has specialized cells too. These cells all serve different functions while still serving a common purpose: supporting the survival of the overall organism.
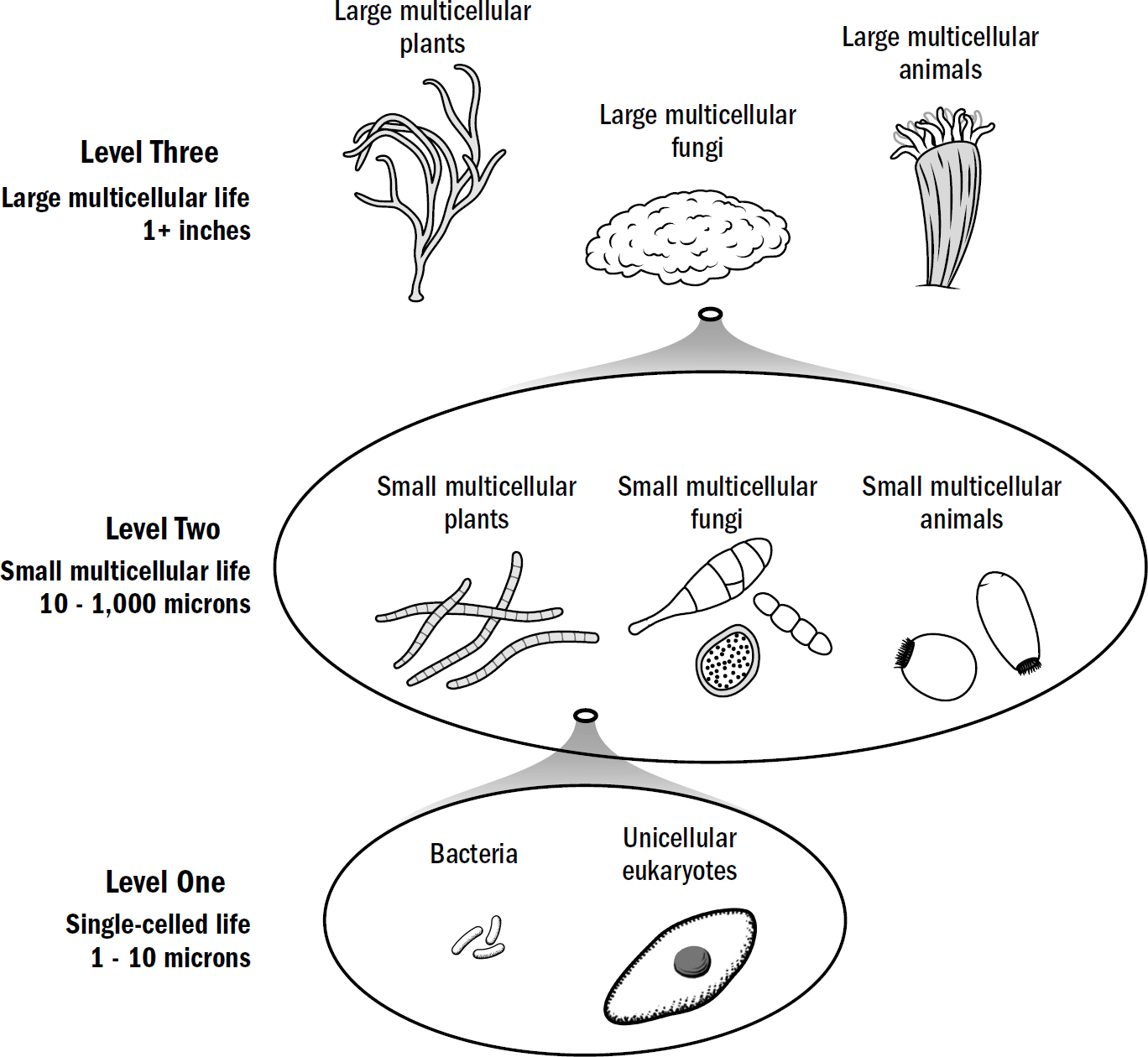
Figure 1.4: Three complexity levels in the ancient sea before brains
Original art by Rebecca Gelernter
These early animals probably wouldn’t resemble what you think of as animals. But they contained something that made them different from all other life at the time: neurons.
The Neuron
What a neuron is and what it does depends on whom you ask. If you ask a biologist, neurons are the primary cells that make up the nervous system. If you ask a machine learning researcher, neurons are the fundamental units of neural networks, little accumulators that perform the basic task of computing a weighted summation of their inputs. If you ask a psychophysicist, neurons are the sensors that measure features of the external world. If you ask a neuroscientist specializing in motor control, neurons are effectors, the controllers of muscles and movement. If you ask other people, you might get a wide range of answers, from “Neurons are little electrical wires in your head” to “Neurons are the stuff of consciousness.” All of these answers are right, carrying a kernel of the whole truth, but incomplete on their own.
The nervous systems of all animals—from worms to wombats—are made up of these stringy odd-shaped cells called neurons. There is an incredible diversity of neurons, but despite this diversity in shapes and sizes, all neurons work the same way. This is the most shocking observation when comparing neurons across species—they are all, for the most part, fundamentally identical. The neurons in the human brain operate the same way as the neurons in a jellyfish. What separates you from an earthworm is not the unit of intelligence itself—neurons—but how these units are wired together.
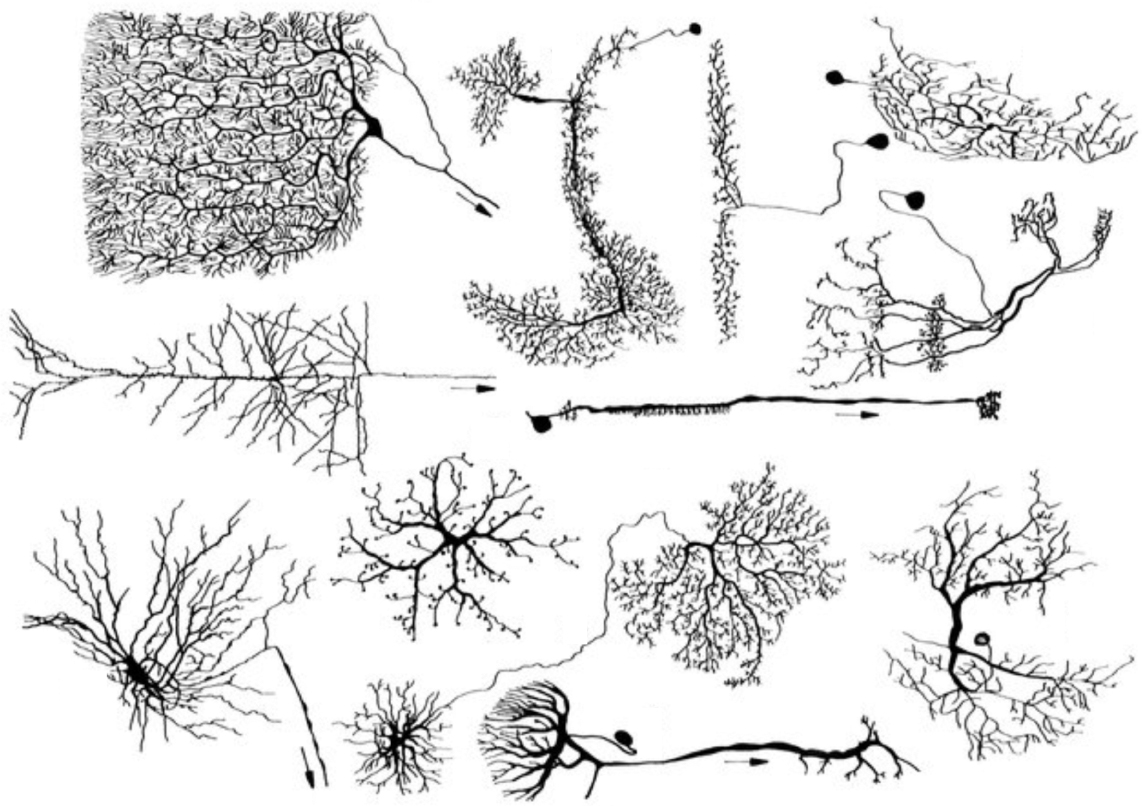
Figure from Reichert, 1990. Used with permission.
Why Fungi Don’t Have Neurons but Animals Do
You aren’t all that different from mold. Despite their appearance, fungi have more in common with animals than they do with plants. While plants survive by photosynthesis, animals and fungi both survive by respiration. Animals and fungi both breathe oxygen and eat sugar; both digest their food, breaking cells down using enzymes and absorbing their inner nutrients; and both share a much more recent common ancestor than either do with plants, which diverged much earlier. At the dawn of multicellularity, fungi and animal life would have been extremely similar. And yet one lineage (animals) went on to evolve neurons and brains, and the other (fungi) did not. Why?
Fungi produce trillions of single-celled spores that float around dormant. If by luck one happens to find itself near dying life, it will blossom into a large fungal structure, growing hairy filaments into the decaying tissue, secreting enzymes, and absorbing the released nutrients. This is why mold always shows up in old food. Fungal spores are all around us, patiently waiting for something to die. Fungi are currently, and likely have always been, Earth’s garbage collectors.
In fact, the formation of an inner cavity for digestion may have been the defining feature of these early animals. Practically every animal alive today develops in the same three initial steps. From a single-celled fertilized egg, a hollow sphere (a blastula) forms; this then folds inward to make a cavity, a little “stomach” (a gastrula). This is true of human embryos
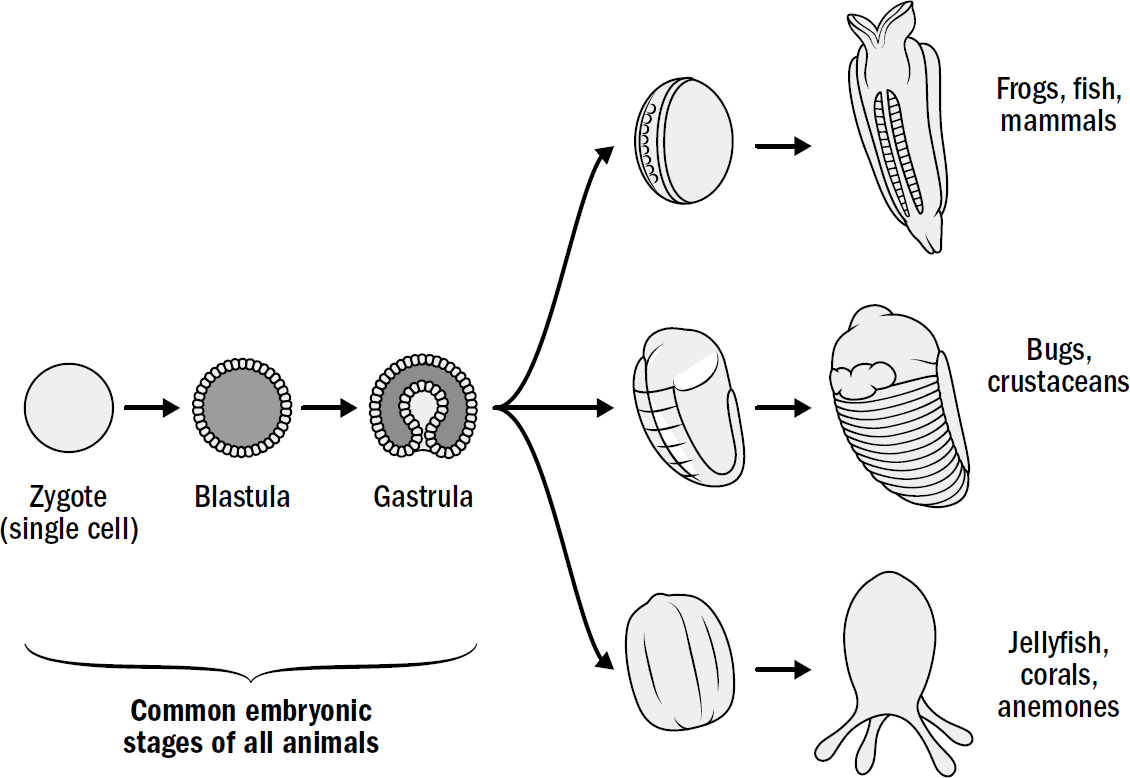
Figure 1.6: Shared developmental stages for all animals
Original art by Rebecca Gelernter
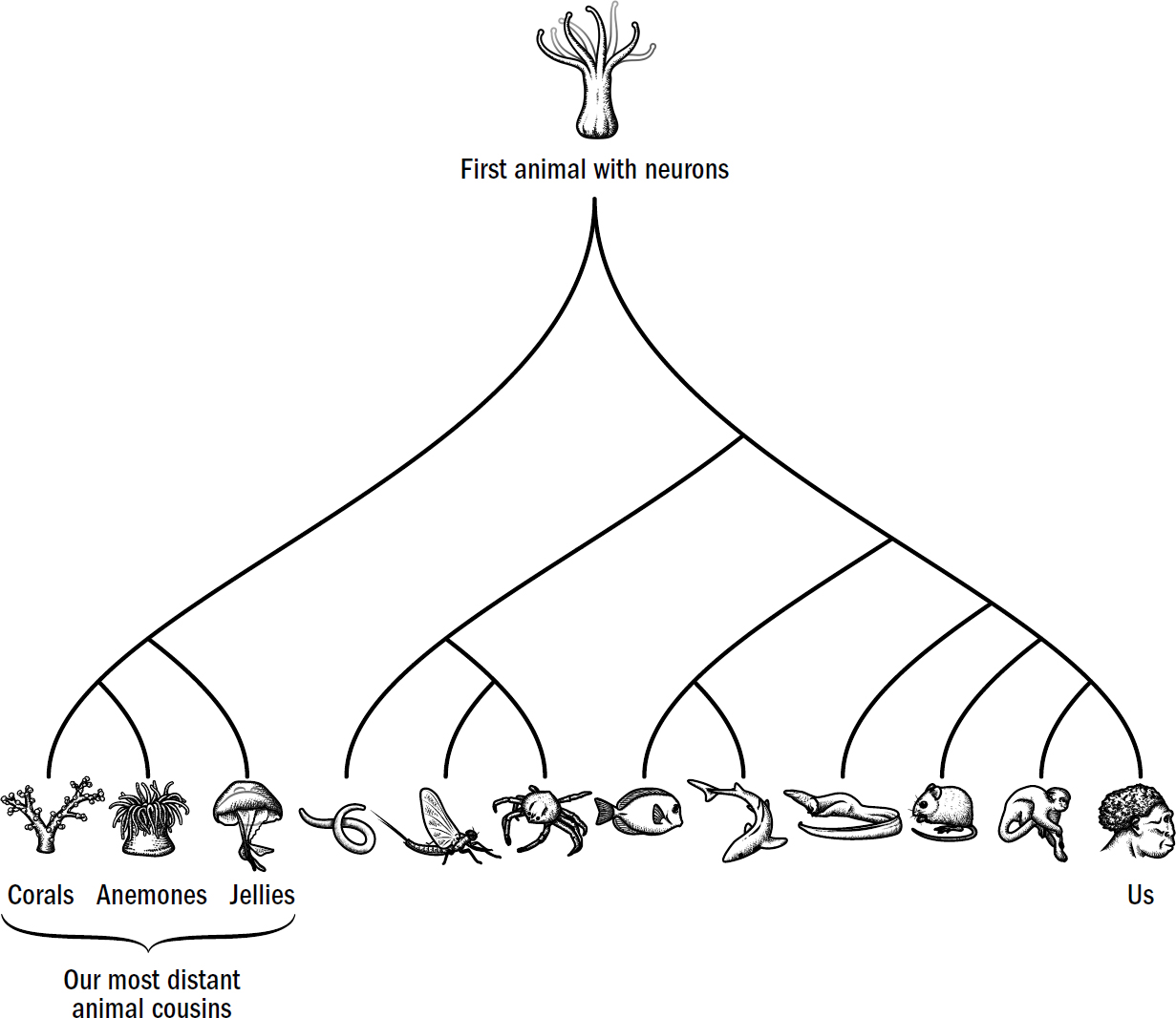
Figure 1.7: Tree of neuron-enabled animals
Original art by Rebecca Gelernter
This coral reflex was not the first or the only way in which multicellular life sensed and responded to the world. Plants and fungi do this just fine without neurons or muscles; plants can orient their leaves toward the sun, and fungi can orient their growth in the direction of food. But still, in the ancient sea at the dawn of multicellularity, this reflex would have been revolutionary, not because it was the first time multicellular life sensed or moved but because it was the first time it sensed and moved with speed and specificity. The movement of plants and fungi takes hours to days; the movement of coral takes seconds.* The movement of plants and fungi is clumsy and inexact; the movement of coral is comparatively very specific—the grasping of prey, opening of the mouth, pulling into the stomach, and closing of the mouth all require a well-timed and accurate orchestration of relaxing some muscles while contracting others. And this is why fungi don’t have neurons and animals do. Although both are large multicellular organisms that feed on other life, only the animal-survival strategy of killing level-two multicellular life requires fast and specific reflexes.* The original purpose of neurons and muscles may have been the simple and inglorious task of swallowing.
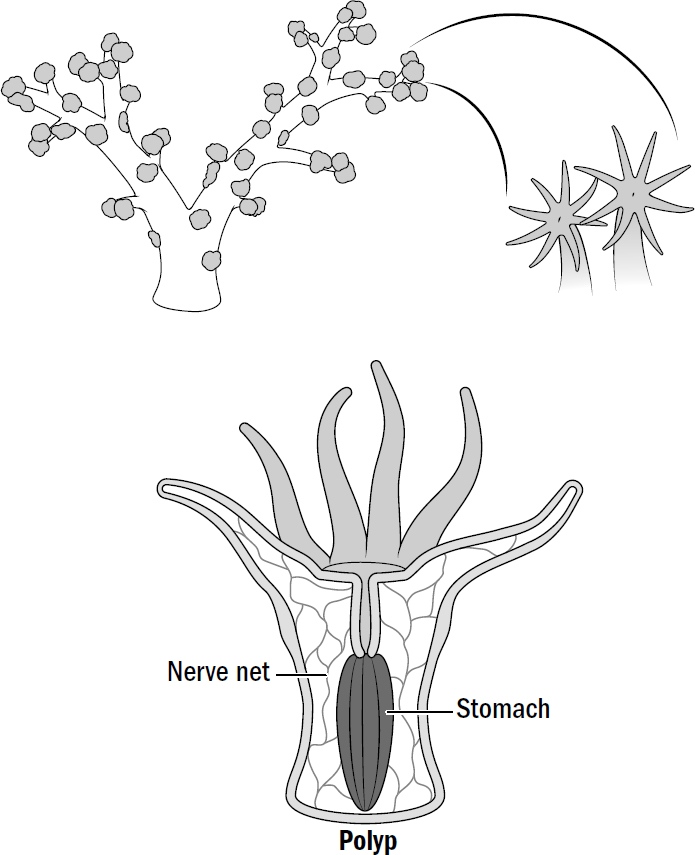
Figure 1.8: Soft coral as a model for early animal life
Original art by Rebecca Gelernter
Edgar Adrian’s Three Discoveries and the Universal Features of Neurons
The scientific journey by which we have come to understand how neurons work has been long and full of
Adrian took a muscle from the neck of a deceased frog and attached a recording device to a single stretch-sensing neuron in the muscle. Such neurons have receptors that are stimulated when muscles are stretched. Adrian then attached various weights to the muscle. The question was: How would the responses of these stretch-sensing neurons change based on the weight placed on the muscle?
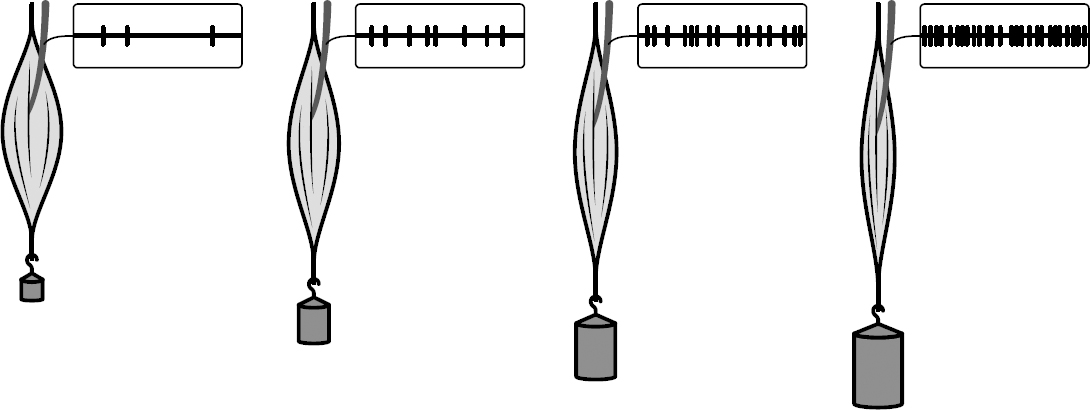
Figure 1.9: Adrian charted the relationship between weight and the number of spikes per second (i.e., the spike rate, or firing rate) elicited in these stretch neurons.
Original art by Rebecca Gelernter
Adrian’s third discovery was the most surprising of all. There is a problem with trying to translate natural variables, such as the pressure of touch or the brightness of light into this language of rate codes. The problem is this: these natural variables have a massively larger range than can be encoded in the firing rate of a neuron.
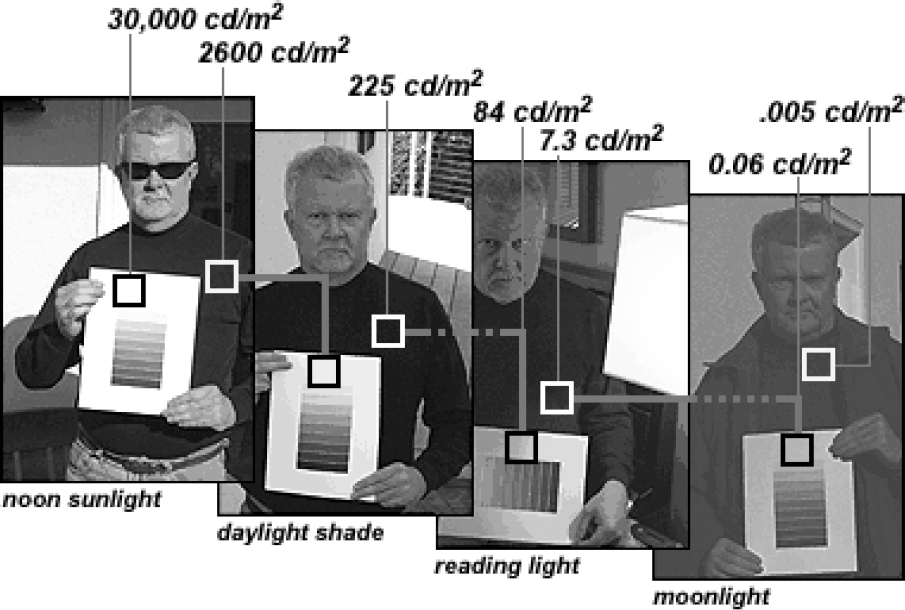
Figure made by B. MacEvoy, 2015. Used with permission (personal correspondence).
This makes rate coding, on its own, untenable. Neurons simply cannot directly encode such a wide range of natural variables in such a small range of firing rates without losing a huge amount of precision. The resulting imprecision would make it impossible to read inside, detect subtle smells, or notice a soft touch.
It turns out that neurons have a clever solution to this problem. Neurons do not have a fixed relationship between natural variables and firing rates. Instead, neurons are always adapting their firing rates to their environment; they are constantly remapping the relationship between variables in the natural world and the language of firing rates. The term neuroscientists use to describe this observation is adaptation; this was Adrian’s third discovery.
In Adrian’s frog muscle experiments, a neuron might fire one hundred spikes in response to a certain weight. But after this first exposure, the neuron quickly adapts; if you apply the same weight shortly after, it might elicit only eighty spikes. And as you keep doing this, the number of spikes continues to decline. This applies in many neurons throughout the brains of animals—the stronger the stimuli, the greater the change in the neural threshold for spiking. In some sense, neurons are more a measurement of relative changes in stimulus strengths, signaling how much the strength of a stimulus changed relative to its baseline as opposed to signaling the absolute value of the stimulus.
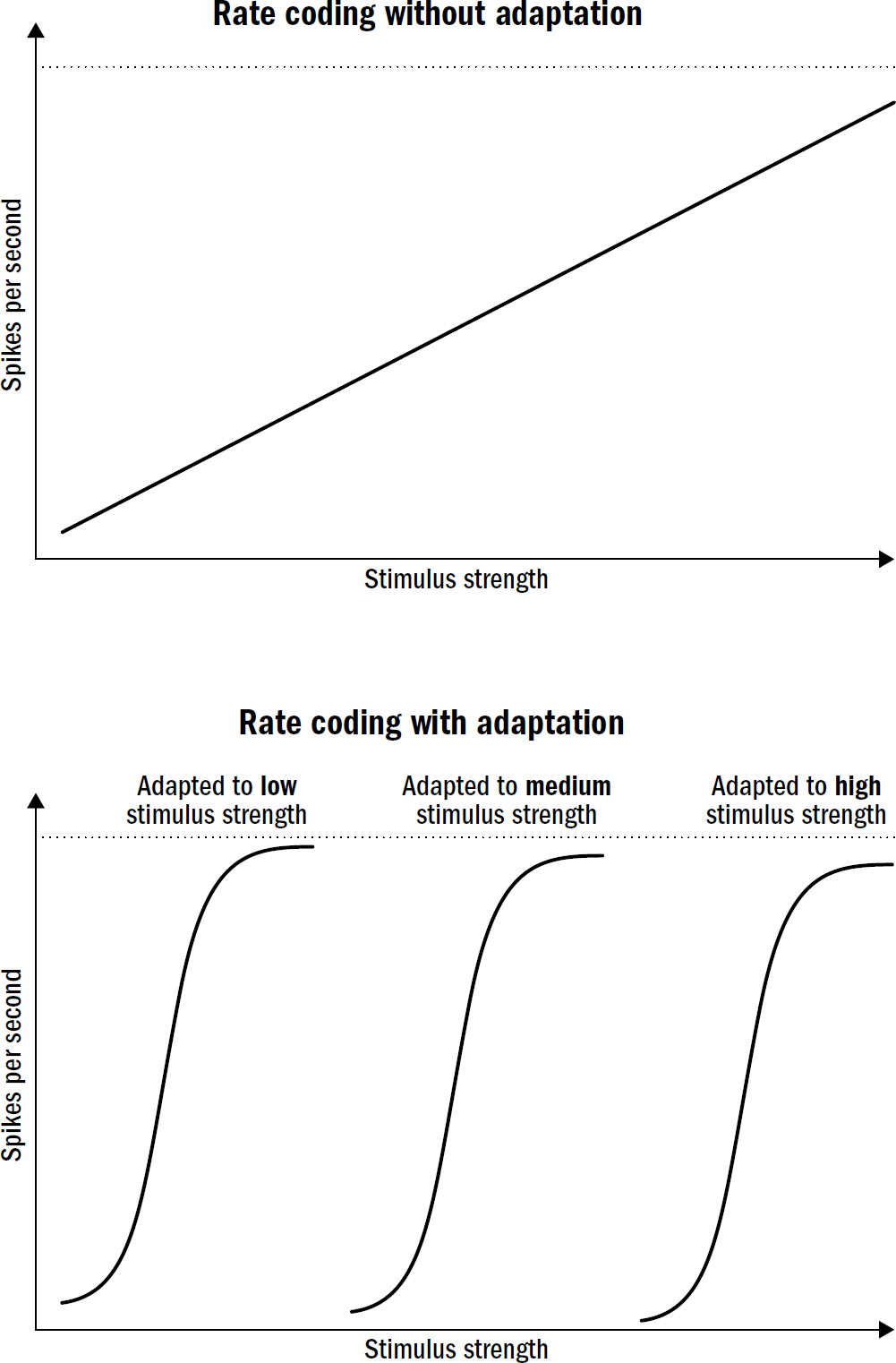
Figure 1.11
Original art by Rebecca Gelernter
Here’s the beauty: Adaptation solves the squishing problem. Adaptation enables neurons to precisely encode a broad range of stimulus strengths despite a limited range of firing rates. The stronger a stimulus is, the more strength will be required to get the neuron to respond similarly next time. The weaker a stimulus is, the more sensitive neurons become.
In the 1950s, John Eccles discovered that neurons come in two main varieties: excitatory neurons and inhibitory neurons. Excitatory neurons release neurotransmitters that excite neurons they connect to, while inhibitory neurons release neurotransmitters that inhibit neurons they connect to. In other words, excitatory neurons trigger spikes in other neurons, while inhibitory neurons suppress spikes in other neurons.
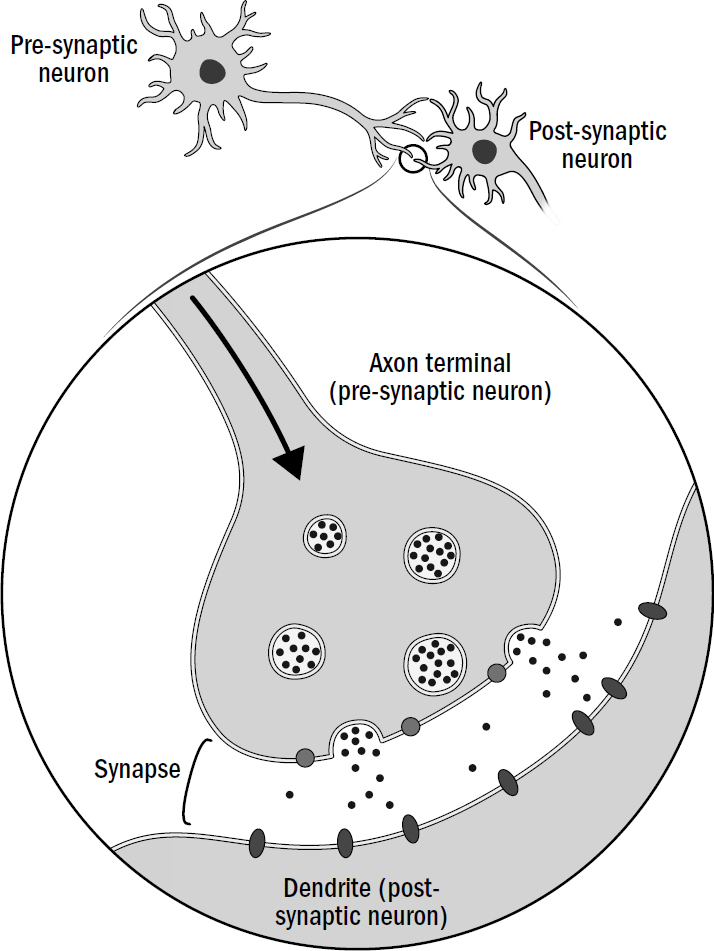
Figure 1.12
Original art by Rebecca Gelernter
These features of neurons—all-or-nothing spikes, rate coding, adaptation, and chemical synapses with excitatory and inhibitory neurotransmitters—are universal across all animals, even in animals that have no brain, such as coral polyps and jellyfish. Why do all neurons share these features? If early animals were, in fact, like today’s corals and anemones, then these aspects of neurons enabled ancient animals to successfully respond to their environment with speed and specificity, something that had become necessary to actively capture and kill level-two multicellular life. All-or-nothing electrical spikes triggered rapid and orchestrated reflexive movements so animals could catch prey in response to even the subtlest of touches or smells. Rate coding enabled animals to modify their responses based on the strengths of a touch or smell. Adaptation enabled animals to adjust the sensory threshold for when spikes are generated, allowing them to be highly sensitive to even the subtlest of touches or smells while also preventing overstimulation at higher strengths of stimuli.
What about inhibitory neurons? Why did they evolve? Consider the simple task of a coral polyp opening or closing its mouth. For its mouth to open, one set of muscles must contract
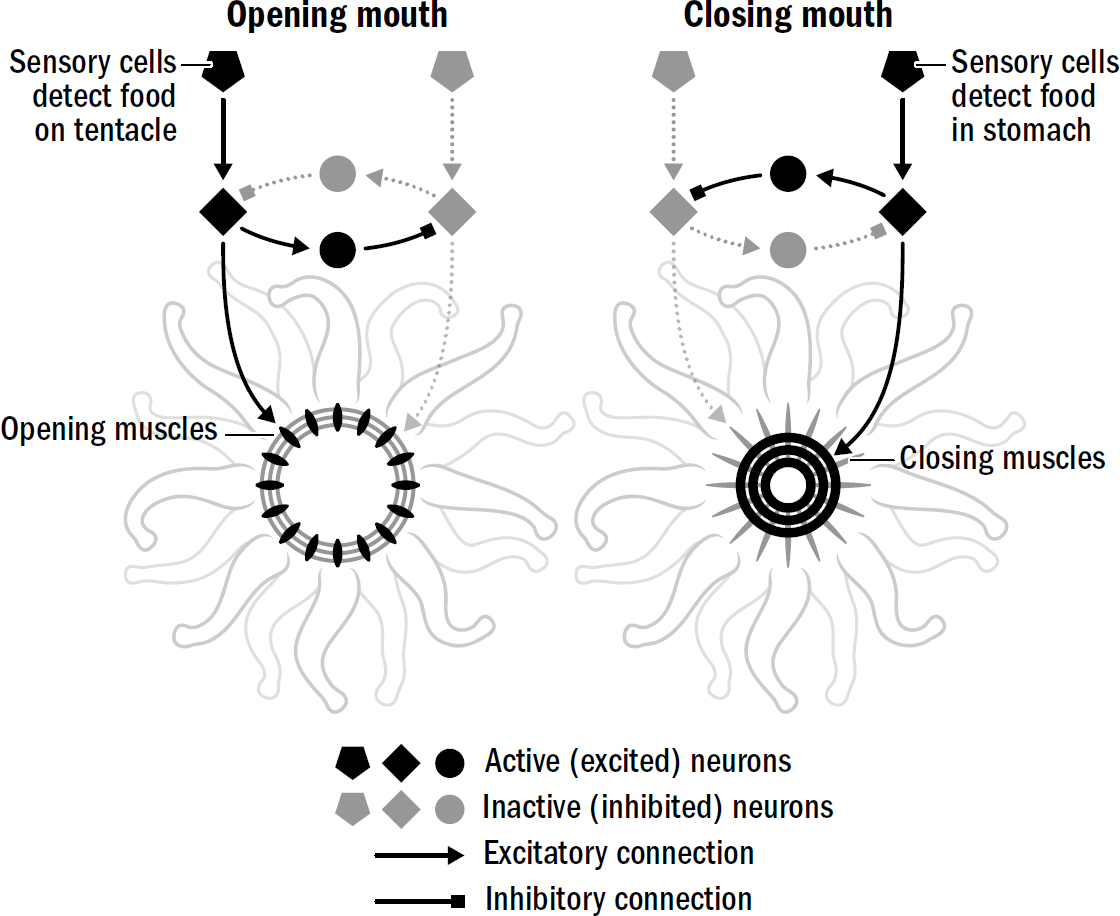
Figure 1.13: The first neural circuit
Original art by Rebecca Gelernter
While the first animals, whether gastrula-like or polyp-like creatures, clearly had neurons, they had no brain. Like today’s coral polyps and jellyfish, their nervous system was what scientists call a nerve net: a distributed web of independent neural circuits implementing their own independent reflexes.
Breakthrough #1
Steering and the First Bilaterians
Your brain 600 million years ago
Original art by Rebecca Gelernter
2
The Birth of Good and Bad
Nature has placed mankind under the governance of two sovereign masters, pain and pleasure.
—JEREMY BENTHAM, AN INTRODUCTION TO THE PRINCIPLES OF MORALS AND LEGISLATION
AT FIRST GLANCE, the diversity of the animal kingdom appears remarkable—from ants to alligators, bees to baboons, and crustaceans to cats, animals seem varied in countless ways. But if you pondered this further, you could just as easily conclude that what is remarkable about the animal kingdom is how little diversity there is. Almost all animals on Earth have the same body plan. They all have a front that contains a mouth, a brain, and the main sensory organs (such as eyes and ears), and they all have a back where waste comes out.
Evolutionary biologists call animals with this body plan bilaterians because of their bilateral symmetry. This is in contrast to our most distant animal cousins—coral polyps, anemones, and jellyfish—which have body plans with radial symmetry; that is, with similar parts arranged around a central axis, without any front or back. The most obvious difference between these two categories is how the animals eat. Bilaterians eat by putting food in their mouths and then pooping out waste products from their butts. Radially symmetrical animals have only one opening—a mouth-butt if you will—which swallows food into their stomachs and spits it out. The bilaterians are undeniably the more proper of the two.
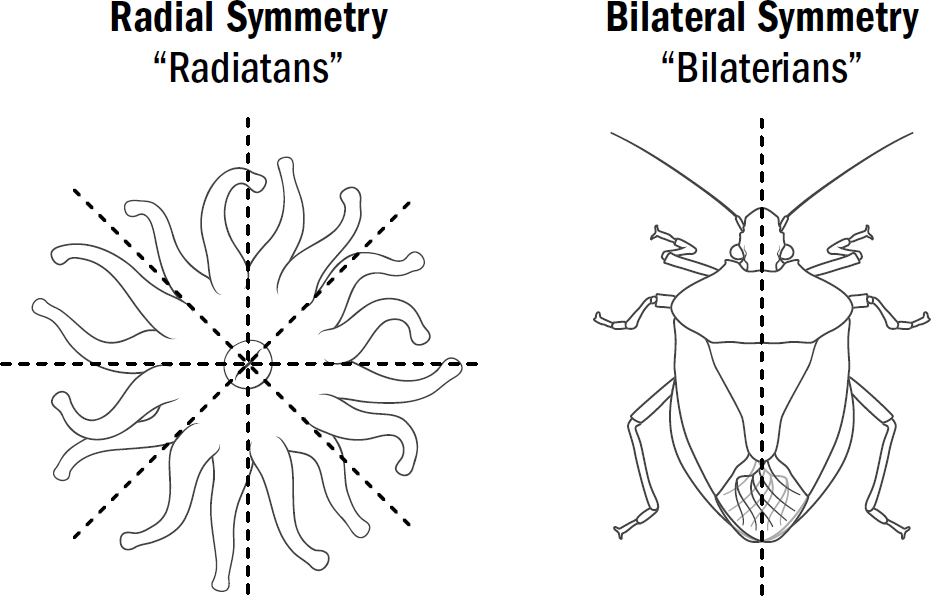
Figure 2.1
Original art by Rebecca Gelernter
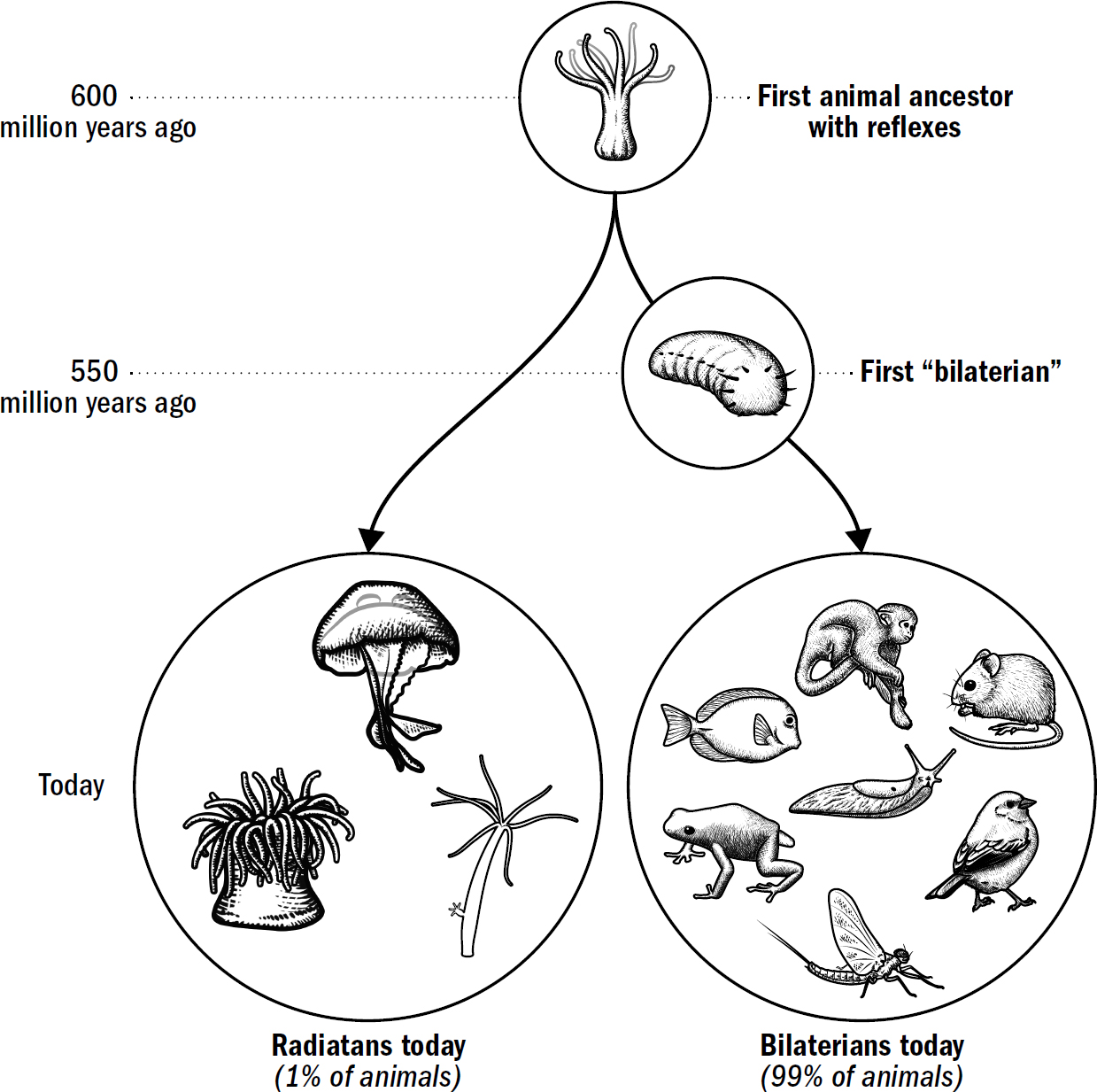
Figure 2.2
Original art by Rebecca Gelernter
The first animals are believed to have been radially symmetric, and yet today, most animal species are bilaterally symmetric. Despite the diversity of modern bilaterians—from worms to humans—they all descend from a single bilaterian common ancestor who lived around 550 million years ago. Why, within this single lineage of ancient animals, did body plans change from radial symmetry to bilateral symmetry?
Radially symmetrical body plans work fine with the coral strategy of waiting for food. But they work horribly for the hunting strategy of navigating toward food. Radially symmetrical body plans, if they were to move, would require an animal to have sensory mechanisms to detect the location of food in any direction and then have the machinery to move in any direction. In other words, they would need to be able to simultaneously detect and move in all different directions. Bilaterally symmetrical bodies make movement much simpler. Instead of needing a motor system to move in any direction, they simply need one motor system to move forward and one to turn. Bilaterally symmetrical bodies don’t need to choose the exact direction; they simply need to choose whether to adjust to the right or the left.
Even modern human engineers have yet to find a better structure for navigation. Cars, planes, boats, submarines, and almost every human-built navigation machine is bilaterally symmetric. It is simply the most efficient design for a movement system. Bilateral symmetry allows a movement apparatus to be optimized for a single direction (forward) while solving the problem of navigation by adding a mechanism for turning.
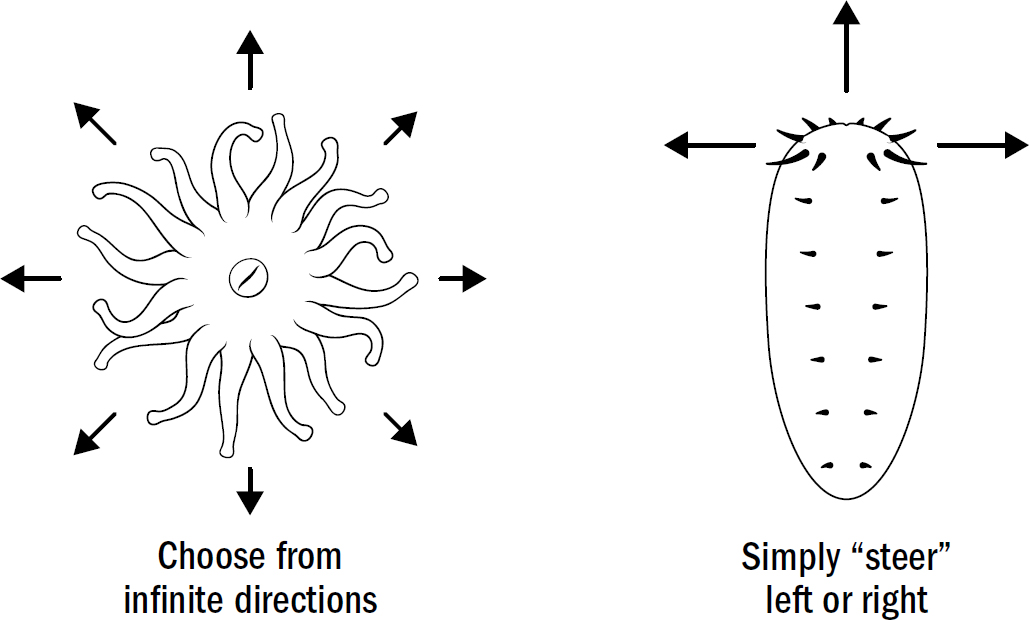
Figure 2.3: Why bilateral symmetry is better for navigation
Original art by Rebecca Gelernter
There is another observation about bilaterians, perhaps the more important one: They are the only animals that have brains. This is not a coincidence. The first brain and the bilaterian body share the same initial evolutionary purpose: They enable animals to navigate by steering. Steering was breakthrough #1.
Navigating by Steering
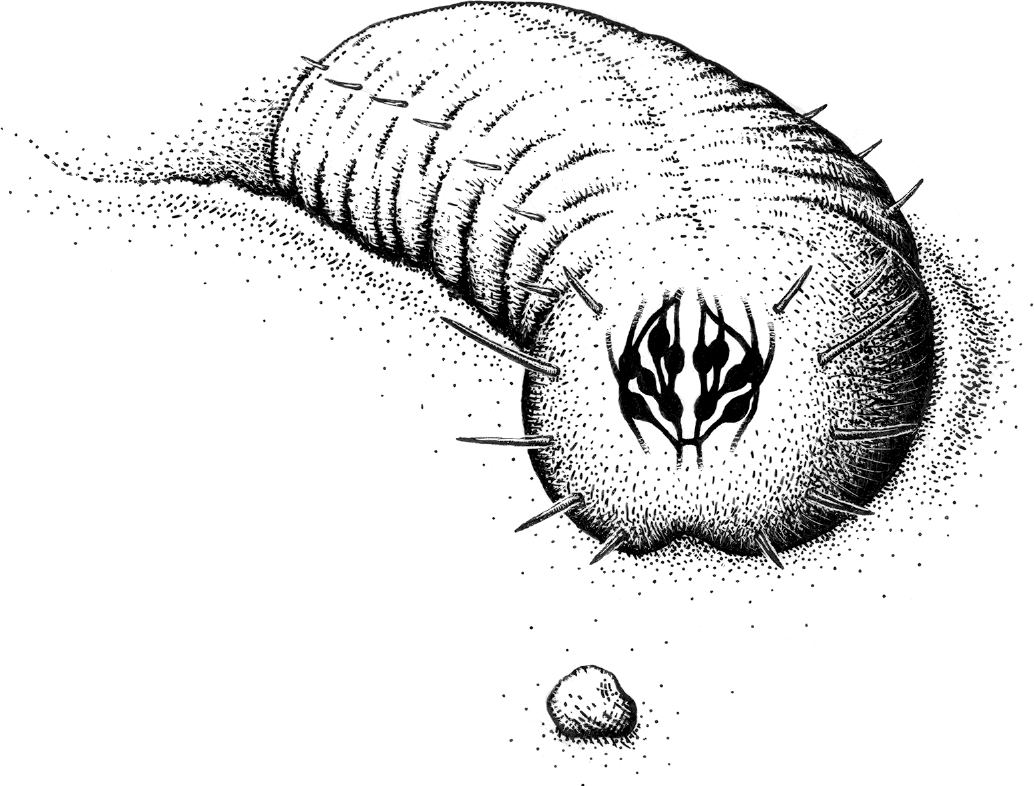
Although we don’t know exactly what the first bilaterians looked like, fossils suggest they were legless wormlike creatures about the size of
Modern nematodes are believed to have remained relatively unchanged since early bilaterians; these creatures give us a window into the inner workings of our wormlike ancestors. Nematodes are almost literally just the basic template of a bilaterian: not much more than a head, mouth, stomach, butt, some muscles, and a brain.
Figure 2.4: The Ediacaran world
Original art by Rebecca Gelernter
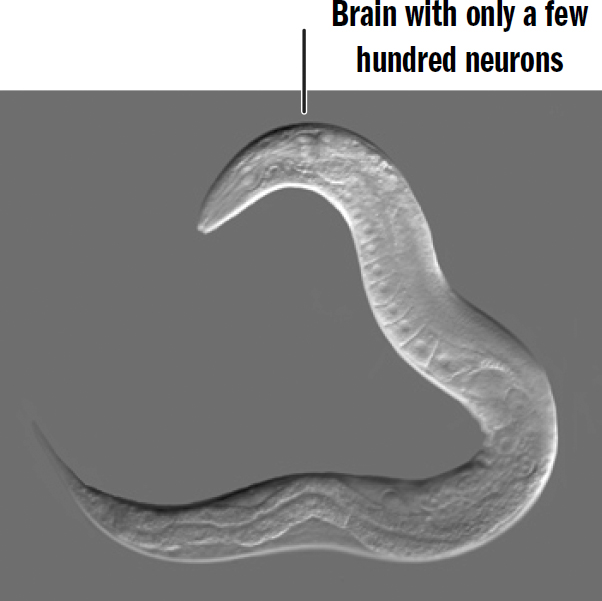
Figure 2.5: The nematode C. elegans
Original art by Rebecca Gelernter
The worm is not using vision; nematodes can’t see. They have no eyes to render any image useful for navigation. Instead, the worm is using smell. The closer it gets to the source of a smell, the higher the concentration of that smell. Worms exploit this fact to find food. All a worm must do is turn toward the direction where the concentration of food particles is increasing, and away from the direction it is decreasing. It is quite elegant how simple yet effective this navigational strategy is. It can be summarized in two rules:
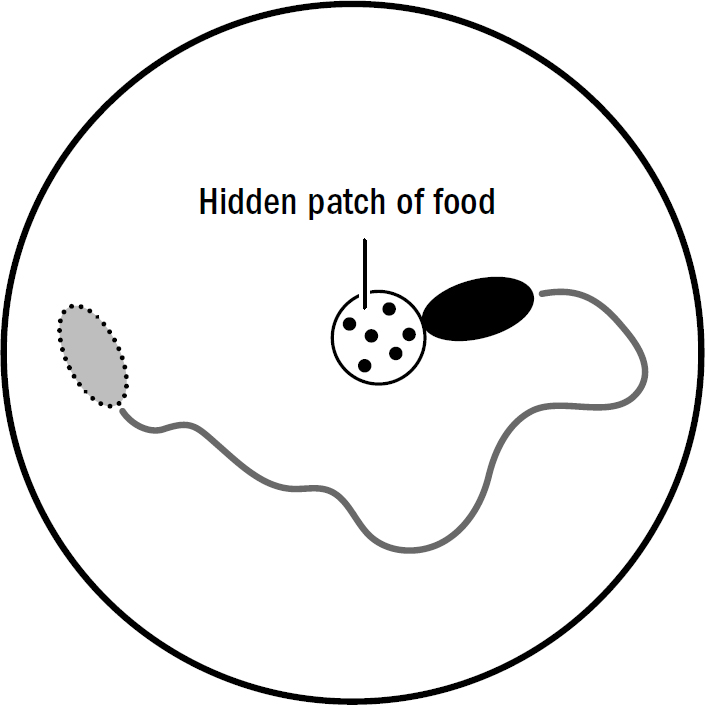
Figure 2.6: Nematode steering toward food
Original art by Rebecca Gelernter
- If food smells increase, keep going forward.
- If food smells decrease, turn.
This was the breakthrough of steering. It turns out that to successfully navigate in the complicated world of the ocean floor, you don’t actually need an understanding of that two-dimensional world. You don’t need an understanding of where you are, where food is, what paths you might have to take, how long it might take, or really anything meaningful about the world. All you need is a brain that steers a bilateral body toward increasing food smells and away from decreasing food smells.
This trick of navigating by steering was not new. Single-celled organisms like bacteria navigate around their environments in a similar way. When a protein receptor on the surface of a bacterium detects a stimulus like light, it can trigger a chemical process within the cell that changes the movement of the cell’s protein propellers, thereby causing it to change its direction. This is how single-celled organisms like bacteria swim toward food sources or away from dangerous chemicals. But this mechanism works only on the scale of individual cells, where simple protein propellers can successfully reorient the entire life-form. Steering in an organism that contains millions of cells required a whole new setup, one in which a stimulus activates circuits of neurons and the neurons activate muscle cells, causing specific turning movements. And so the breakthrough that came with the first brain was not steering per se, but steering on the scale of multicellular organisms.
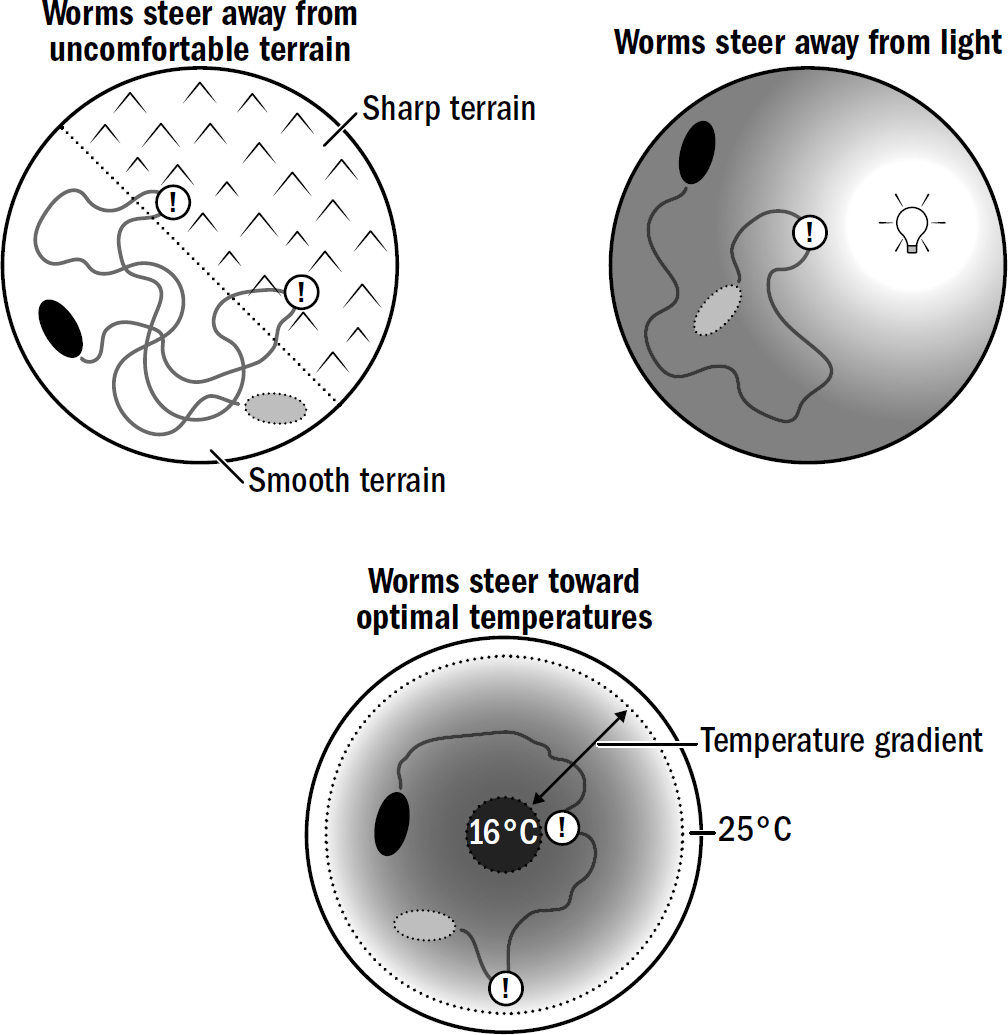
Figure 2.7: Examples of steering decisions made by simple bilaterians like nematodes and flatworms.
Original art by Rebecca Gelernter
The First Robot
In the 1980s and 1990s a schism emerged in the artificial intelligence community. On one side were those in the symbolic AI camp, who were focused on decomposing human intelligence into its constituent parts in an attempt to imbue AI systems with our most cherished skills: reasoning, language, problem solving, and logic. In opposition were those in the behavioral AI camp, led by the roboticist Rodney Brooks at MIT, who believed the symbolic approach was doomed to fail because “we will never understand how to decompose human level intelligence until we’ve had a lot of practice with simpler level intelligences.”
Brooks’s argument was partly based on evolution: it took billions of years before life could simply sense and respond to its environment; it took another five hundred million years of tinkering for brains to get good at motor skills and navigation; and only after all of this hard work did language and logic appear. To Brooks, compared to how long it took for sensing and moving to evolve, logic and language appeared in a blink of an eye. Thus he concluded that “language . . . and reason, are all pretty simple once the essence of being and reacting are available. That essence is the ability to move around in a dynamic environment, sensing the surroundings to a degree sufficient to achieve the necessary maintenance of life and reproduction. This part of intelligence is where evolution has concentrated its time—it is much harder.”
By trying to skip simple planes and directly build a 747, they risked completely misunderstanding the principles of how planes work (pitched seats, paned windows, and plastics are the wrong things to focus on). Brooks believed the exercise of trying to reverse-engineer the human brain suffered from this same problem. A better approach was to “incrementally build up the capabilities of intelligence systems, having complete systems at each step.” In other words, to start as evolution did, with simple brains, and add complexity from there.
Many do not agree with Brooks’s approach, but whether you agree with him or not, it was Rodney Brooks who, by any reasonable account, was the first to build a commercially successful domestic robot; it was Brooks who made the first small step toward Rosey. And this first step in the evolution of commercial robots has parallels to the first step in the evolution of brains. Brooks, too, started with steering.

Photograph by Larry D. Moore in 2006. Picture published on Wikipedia at https://en.wikipedia.org/wiki/Roomba.
The Roomba could clean all the nooks and crannies of your floor by simply moving around randomly, steering away from obstacles when it bumped into them, and steering toward its charging station when it was low on battery. Whenever the Roomba hit a wall, it would perform a random turn and try to move forward again. When it was low on battery, the Roomba searched for a signal from its charging station, and when it detected the signal, it simply turned in the direction where the signal was strongest, eventually making it back to its charging station.
The navigational strategies of the Roomba and first bilaterians were not identical. But it may not be a coincidence that the first successful domestic robot contained an intelligence not so unlike the intelligence of the first brains. Both used tricks that enabled them to navigate a complex world without actually understanding or modeling that world.
While others remained stuck in the lab working on million-dollar robots with eyes and touch and brains that attempted to compute complicated things like maps and movements, Brooks built the simplest possible robot, one that contained hardly any sensors and that computed barely anything at all. But the market, like evolution, rewards three things above all: things that are cheap, things that work, and things that are simple enough to be discovered in the first place.
While steering might not inspire the same awe as other intellectual feats, it was surely energetically cheap, it worked, and it was simple enough for evolutionary tinkering to stumble upon it. And so it was here where brains began.
Valence and the Inside of a Nematode’s Brain
Around the head of a nematode are sensory neurons, some of which respond to light, others to touch, and others to specific chemicals. For steering to work, early bilaterians needed to take each smell, touch, or other stimulus they detected and make a choice: Do I approach this thing, avoid this thing, or ignore this thing?
The breakthrough of steering required bilaterians to categorize the world into things to approach (“good things”) and things to avoid (“bad things”). Even a Roomba does this—obstacles are bad; charging station when low on battery is good. Earlier radially symmetric animals did not navigate, so they never had to categorize things in the world like this.
When animals categorize stimuli into good and bad, psychologists and neuroscientists say they are imbuing stimuli with valence. Valence is the goodness or badness of a stimulus. Valence isn’t about a moral judgment; it’s something far more primitive: whether an animal will respond to a stimulus by approaching it or avoiding it. The valence of a stimulus is, of course, not objective; a chemical, image, or temperature, on its own, has no goodness or badness. Instead, the valence of a stimulus is subjective, defined only by the brain’s evaluation of its goodness or badness.
How does a nematode decide the valence of something it perceives? It doesn’t first observe something, ponder it, then decide its valence. Instead, the sensory neurons around its head directly signal the stimulus’s valence. One group of sensory neurons are, effectively, positive-valence neurons; they are directly activated by things nematodes deem good (such as food smells). Another group of sensory neurons are, effectively, negative-valence neurons; they are directly activated by things nematodes deem bad (such as high temperatures, predator smells, bright light).
In nematodes, sensory neurons don’t signal objective features of the surrounding world—they encode steering votes for how much a nematode wants to steer toward or away from something. In more complex bilaterians, such as humans, not all sensory machinery is like this—the neurons in your eyes detect features of images; the valence of the image is computed elsewhere. But it seems that the first brains began with sensory neurons that didn’t care to measure objective features of the world and instead cast the entirety of perception through the simple binary lens of valence.
Consider how a nematode uses this circuit to find food. Nematodes have positive-valence neurons that trigger forward movement when the concentration of a food smell increases. As we saw in the sensory neurons in the nerve net of earlier animals, these neurons quickly adapt to baseline levels of smells. This enables these valence neurons to signal changes across a wide range of smell concentrations. These neurons will generate a similar number of spikes whether a smell concentration goes from two to four parts or from one hundred to two hundred parts. This enables valence neurons to keep nudging the nematode in the right direction. It is the signal for Yes, keep going! from the first whiff of a faraway food smell all the way to the food source.
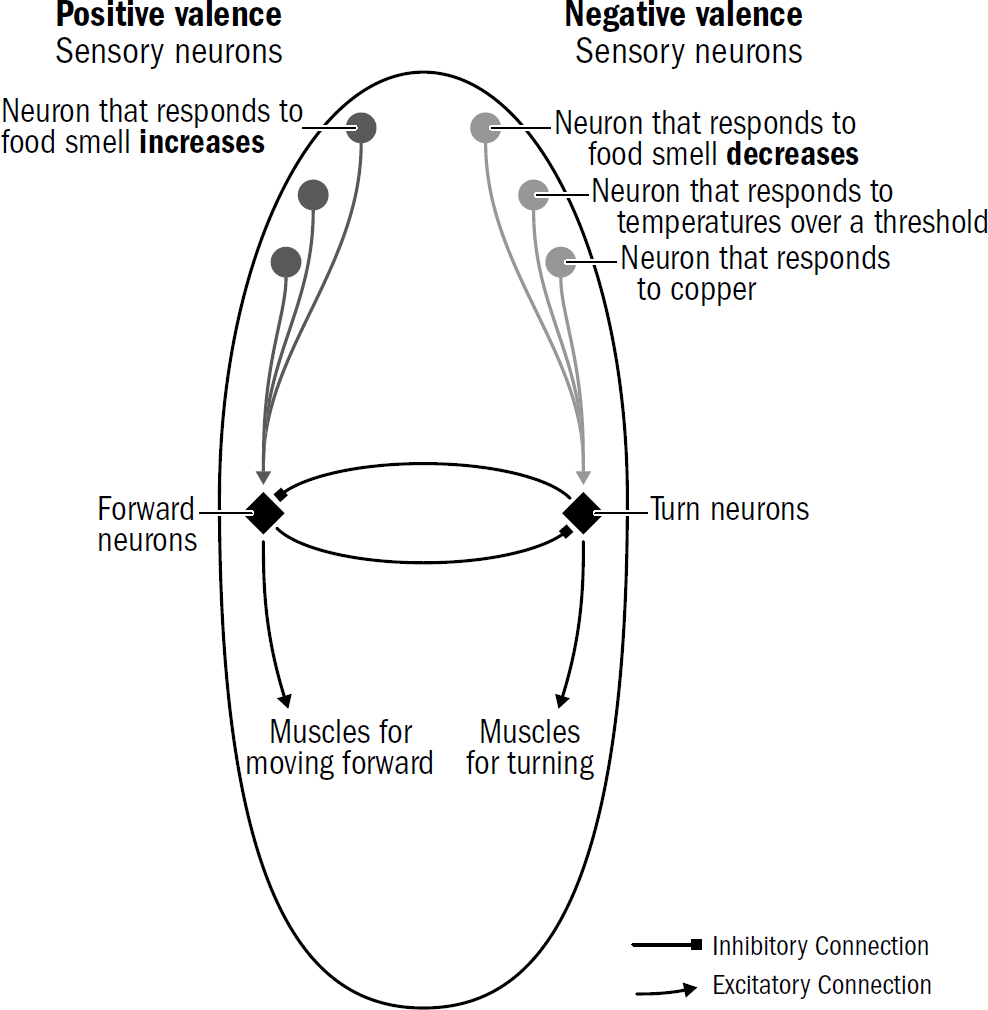
Figure 2.9: A simplified schematic of the wiring of the first brain
Original art by Rebecca Gelernter
This use of adaptation is an example of evolutionary innovations enabling future innovations. Steering toward food in early bilaterians was possible only because adaptation had already evolved in earlier radially symmetric animals. Without adaptation, valence neurons would be either too sensitive (and continuously misfire when smells are too close) or not sensitive enough (unable to detect faraway smells).
At this point, new navigational behaviors could emerge simply by modifying the conditions under which different valence neurons get excited. For example, consider how nematodes navigate toward optimal temperatures. Temperature navigation requires some additional cleverness relative to the simple steering toward smells: the decreasing concentration of a food smell is always bad, but the decreasing temperature of an environment is bad only if a nematode is already too cold. If a nematode is hot, then decreasing temperature is good. A warm bath is miserable in a scorching summer but heavenly in a cold winter. How did the first brains manage to treat temperature fluctuations differently depending on the context?
The Problem of Trade-Offs
Steering in the presence of multiple stimuli presented a problem: What happens if different sensory cells vote for steering in opposite directions? What if a nematode smells both something yummy and something dangerous at the same time?
At low levels of copper, most nematodes cross the barrier; at intermediate levels of copper, only some do; at high levels of copper, no nematodes are willing to cross the barrier.
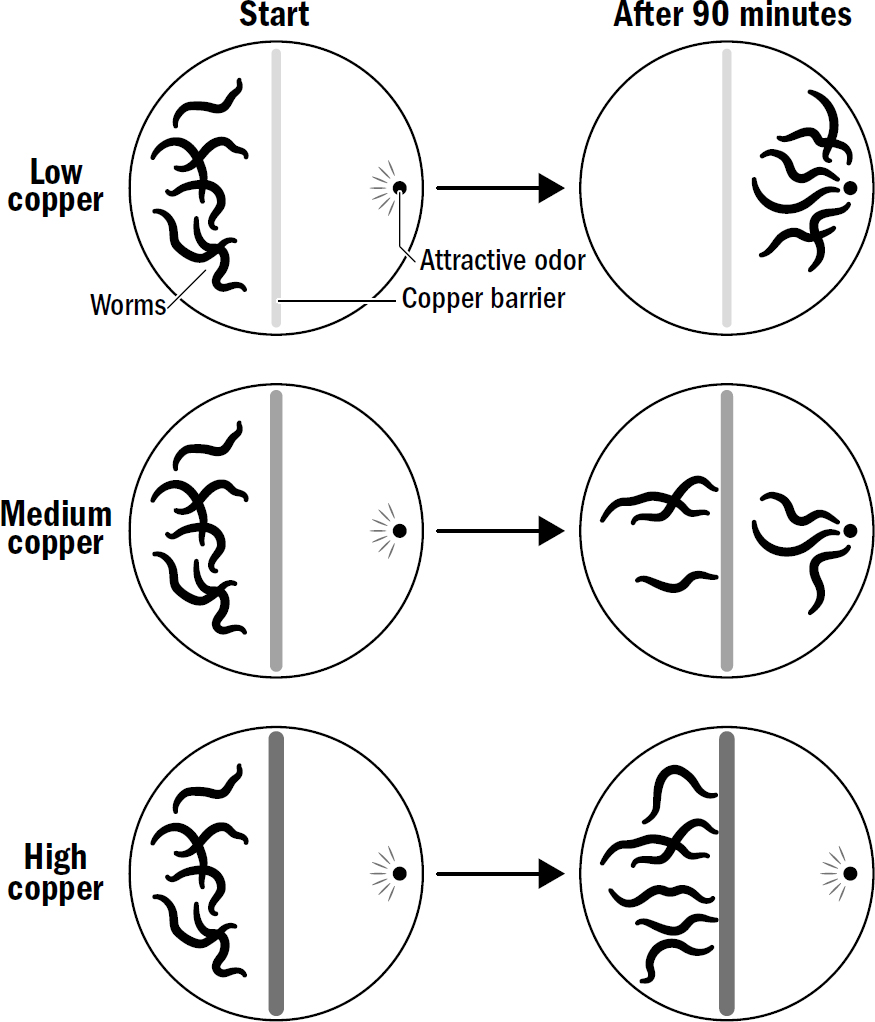
Figure 2.10
Original art by Rebecca Gelernter
This requirement of integrating input across sensory modalities was likely one reason why steering required a brain and could not have been implemented in a distributed web of reflexes like those in a coral polyp. All these sensory inputs voting for steering in different directions had to be integrated together in a single place to make a single decision; you can go in only one direction at a time. The first brain was this mega-integration center—one big neural circuit in which steering directions were selected.
This is another example of how past innovations enabled future innovations. Just as a bilaterian cannot both go forward and turn at the same time, a coral polyp cannot both open and close its mouth at the same time. Inhibitory neurons evolved in earlier coral-like animals to enable these mutually exclusive reflexes to compete with each other so that only one reflex could be selected at a time; this same mechanism was repurposed in early bilaterians to enable them to make trade-offs in steering decisions. Instead of deciding whether to open or close one’s mouth, bilaterians used inhibitory neurons to decide whether to go forward or turn.
Are You Hungry?
The brain’s ability to rapidly flip the valence of a stimulus depending on internal states is ubiquitous. Compare the salivary ecstasy of the first bite of your favorite dinner after a long day of skipped meals to the bloated nausea of the last bite after eating yourself into a food coma. Within mere minutes, your favorite meal can transform from God’s gift to mankind to something you want nowhere near you.
Internal states are present in a Roomba as well. A Roomba will ignore the signal from its home base when it is fully charged. In this case, the signal from the home base can be said to have neutral valence. When the Roomba’s internal state changes to one where it is low on battery, the signal from home base shifts to having positive valence: the Roomba will no longer ignore the signal from its charging station and will steer toward it to replenish its battery.
Steering requires at least four things: a bilateral body plan for turning, valence neurons for detecting and categorizing stimuli into good and bad, a brain for integrating input into a single steering decision, and the ability to modulate valence based on internal states. But still, evolution continued tinkering. There is another trick that emerged in early bilaterian brains, a trick that further bolstered the effectiveness of steering. That trick was the early kernel of what we now call emotion.
3
The Origin of Emotion
THE BLOOD-BOILING FURY you feel when you hear a friend defend the opposite political party’s most recent gaffe, while hard to define emotionally—perhaps some complex mixture of anger, disappointment, betrayal, and shock—is clearly a bad mood. The tingly serenity you feel when you lie on a warm sunny beach, also hard to define exactly, is still clearly a good mood. Valence exists not only in our assessment of external stimuli but also in our internal states.
Our internal states are not only imbued with a level of valence, but also a degree of arousal. Blood-boiling fury is not only a bad mood but an aroused bad mood. Different from an unaroused bad mood, like depression or boredom. Similarly, the tingly serenity of lying on a warm beach is not only a good mood but a good mood with low arousal. Different from the highly arousing good mood produced by getting accepted to college or riding a roller coaster (if you like that sort of thing).
Emotions are complicated. Defining and categorizing specific emotions is a perilous business, ripe with cultural bias. In German, there is a word, sehnsucht, that roughly translates to the emotion of wanting a different life; there is no direct English translation. In Persian, the word ænduh expresses the concepts of regret and grief simultaneously; in Dargwa, the word dard expresses the concepts of anxiety and grief simultaneously. In English we have
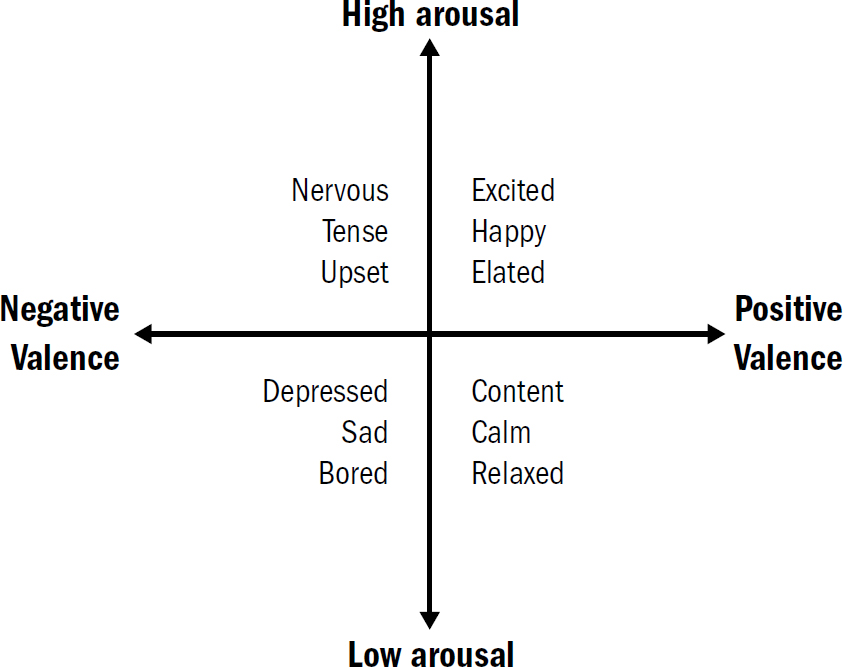
Figure 3.1: The affective states of humans
Original art by Rebecca Gelernter
The universality of affect stretches beyond the bounds of humanity; it is found across the animal kingdom. Affect is the ancient seed from which modern emotions sprouted. But why did affect evolve?
Steering in the Dark
Even nematodes with their minuscule nervous systems have affective states, albeit incredibly simple ones. Nematodes express different levels of arousal: When well fed, stressed, or ill, they hardly move at all and become unresponsive to external stimuli (low arousal); when hungry, detecting food, or sniffing predators, they will continually swim around (high arousal). The affective states of nematodes also express different levels of valence. Positive-valenced stimuli facilitate feeding, digestion, and reproductive activities (a primitive good mood), while negative-valenced stimuli inhibit all of these (a primitive bad mood).
Put these different levels of arousal and valence together and you get a primitive template of affect. Negative-valenced stimuli trigger a behavioral repertoire of fast swimming and infrequent turns, which can be thought of as the most primitive version of an aroused bad mood (which is often called the state of escaping), while the detection of food triggers a repertoire of slow swimming and frequent turns, which can be thought of as the most primitive version of an aroused good mood (which is often called the state of exploiting). Escaping leads worms to rapidly change location; exploiting leads worms to search their local surroundings (to exploit their surroundings for food). Although nematodes don’t share the same complexity of emotions as humans—they do not know the rush of young love or the bittersweet tears of sending a child off to college—they clearly show the basic template of affect. These incredibly simple affective states of nematodes offer a clue as to why affect evolved in the first place.
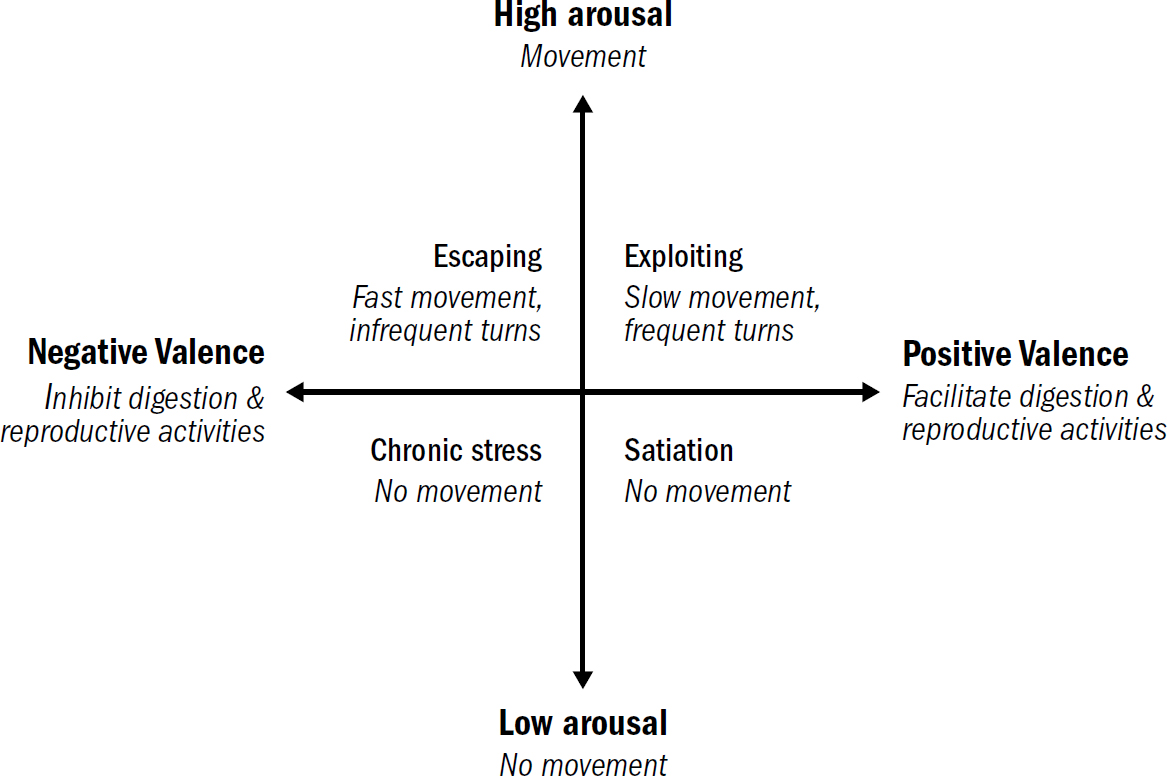
Figure 3.2: The affective states of nematodes
Original art by Rebecca Gelernter
Suppose you put an unfed nematode in a big petri dish with a hidden patch of food. Even if you obscure any food smell for the nematode to steer toward, the nematode won’t just dumbly sit waiting for a whiff of food. The nematode will rapidly swim and relocate itself; in other words, it will escape. It does this because one thing that triggers escape is hunger. When the nematode happens to stumble upon the hidden food, it will immediately slow down and start rapidly turning, remaining in the same general location that it found food—it will shift from escaping to exploiting. Eventually, after eating enough food, the worm will stop moving and become immobile and unresponsive. It will shift to satiation.
Scientists tend to shy away from the term affective states in simple bilaterians such as nematodes and instead use the safer term behavioral states; this avoids the suggestion that nematodes are, in fact, feeling anything. Conscious experience is a philosophical quagmire we will only briefly touch on later. Here, at least, this issue can be sidestepped entirely; the conscious experience of affect—whatever it is and however it works—likely evolved after the raw underlying mechanisms of affect. This can be seen even in humans—the parts of the human brain that generate the experience of negative or positive affective states are evolutionarily newer and distinct from the parts of the brain that generate the reflexive avoidance and approach responses.
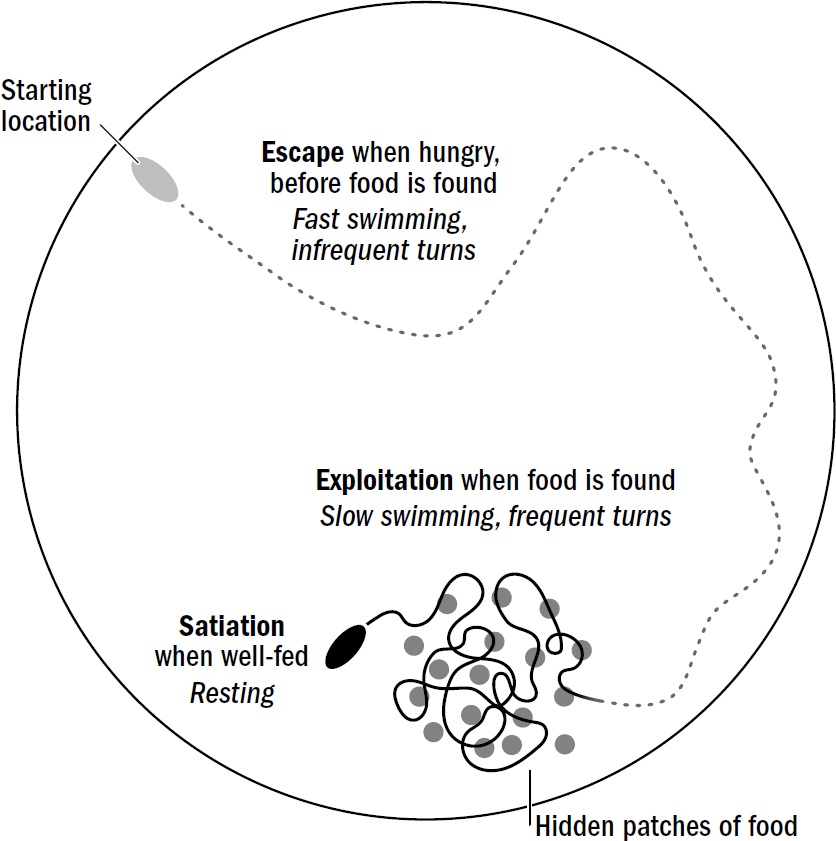
Figure 3.3
Original art by Rebecca Gelernter
Sensory stimuli, especially the simple ones detected by nematodes, offer transient clues, not consistent certainties, of what exists in the real world. In the wild, outside of a scientist’s petri dish, food does not make perfectly distributed smell gradients—water currents can distort or even completely obscure smells, disrupting a worm’s ability to steer toward food or away from predators. These persistent affective states are a trick to overcome this challenge: If I detect a passing sniff of food that quickly fades, it is likely that there is food nearby even if I no longer smell it. Therefore, it is more effective to persistently search my surroundings after encountering food, as opposed to only responding to food smells in the moment that they are detected. Similarly, a worm passing through an area full of predators won’t experience a constant smell of predators but rather catch a transient hint of one nearby; if a worm wants to escape, it is a good idea to persistently swim away even after the smell has faded.
Like a pilot trying to fly a plane while looking through an opaque or obscured window, she would have no choice but to learn to fly in the darkness, using only the clues offered by the flickers of the outside world. Similarly, worms had to evolve a way to “steer in the dark”—to make steering decisions in the absence of sensory stimuli. The first evolutionary solution was affect, behavioral repertoires that can be triggered by external stimuli but persist long after they have faded.
This feature of steering shows up even in a Roomba. Indeed, Roombas were designed to have different behavioral states for the same reason. Normally, they explore rooms by moving around randomly. However, if a Roomba encounters a patch of dirt, it activates Dirt Detect, which changes its repertoire; it begins turning in circles in a local area. This new repertoire is triggered by the detection of dirt but persists for a time even after dirt is no longer detected. Why was the Roomba designed to do this? Because it works—detecting a patch of dirt in one location is predictive of nearby patches of dirt. Thus, a simple rule to improve the speed of getting all the dirt is to shift toward local search for a time after detecting dirt. This is exactly the same reason nematodes evolved to shift their behavioral state from exploration to exploitation after encountering food and locally search their surroundings.
Dopamine and Serotonin
The brain of a nematode generates these affective states using chemicals called neuromodulators. Two of the most famous neuromodulators are dopamine and serotonin. Antidepressants, antipsychotics, stimulants, and psychedelics all exert their effects by manipulating these neuromodulators. Many psychiatric conditions, including depression, obsessive-compulsive disorder, anxiety, post-traumatic stress disorder, and schizophrenia are believed to be caused, at least in part, by imbalances in neuromodulators. Neuromodulators evolved long before humans appeared; they began their connection to affect as far back as the first bilaterians.
Unlike excitatory and inhibitory neurons, which have specific, short-lived effects on only the specific neurons they connect to, neuromodulatory neurons have subtle, long-lasting, and wide-ranging effects on many neurons. Different neuromodulatory neurons release different neuromodulators—dopamine neurons release dopamine, serotonin neurons release serotonin. And neurons throughout an animal’s brain have different types of receptors for different types of neuromodulators—neuromodulators can gently inhibit some neurons while simultaneously activating others; they can make some neurons more likely to spike while making others less likely to spike; they can make some neurons more sensitive to activation while dulling the responses of others. They can even accelerate or slow down the process of adaptation. Put all these effects together, and these neuromodulators can tune the neural activity across the entire brain. It is the balance of these different neuromodulators that determines a nematode’s affective state.
The simple brain of the nematode offers a window into the first, or at least very early, functions of dopamine and serotonin. In the nematode, dopamine is released when food is detected around the worm, whereas serotonin is released when food is detected inside the worm. If dopamine is the something-good-is-nearby chemical, then serotonin is the something-good-is-actually-happening chemical. Dopamine drives the hunt for food; serotonin drives the enjoyment of it once it is being eaten.
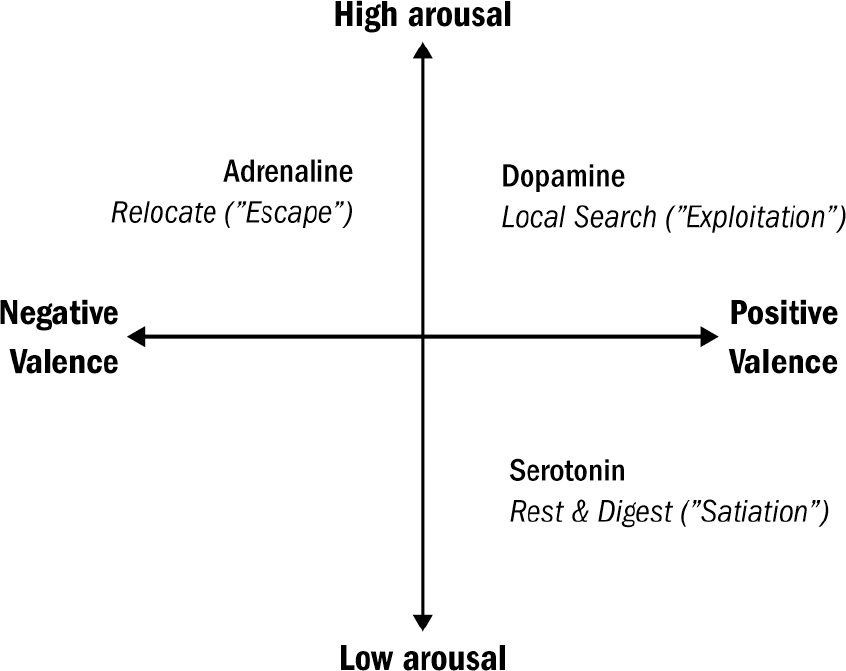
Figure 3.4: Role of neuromodulators in affective states of first bilaterians
Original art by Rebecca Gelernter
And crucially, all these neuromodulatory neurons—like valence neurons—are also sensitive to internal states. Dopamine neurons are more likely to respond to food cues when an animal is hungry.
This connection between dopamine and reward has caused dopamine to be—incorrectly—labeled the “pleasure chemical.” Kent Berridge, a neuroscientist at the University of Michigan, came up with a experimental paradigm to explore the relationship between dopamine and pleasure. Rats, like humans, make distinct facial expressions when tasting things they like, such as yummy sugar pellets, and things they don’t like, such as bitter liquid. A baby will smile when tasting warm milk and spit when tasting bitter water; rats will lick their lips when they taste yummy food and gape their mouths and shake their heads when they taste gross food. Berridge realized he could use the frequency of these different facial reactions as a proxy for identifying pleasure in rats.

Images from Kent Berridge (personal correspondence). Used with permission.
To the surprise of many, Berridge found that increasing dopamine levels in the brains of rats had no impact on the degree and frequency of their pleasurable facial expressions to food. While dopamine will cause rats to consume ridiculous amounts of food, the rats do not indicate they are doing so because they like the food more. Rats do not express a higher number of pleasurable lip smacks. If anything, they express more disgust with the food, despite eating more of it. It is as if rats can’t stop eating even though they no longer enjoy it.
Dopamine is not a signal for pleasure itself; it is a signal for the anticipation of future pleasure. Heath’s patients weren’t experiencing pleasure; to the contrary, they often became extremely frustrated at their inability to satisfy the incredible cravings the button produced.
Berridge proved that dopamine is less about liking things and more about wanting things. This discovery makes sense given the evolutionary origin of dopamine. In nematodes, dopamine is released when they are near food but not when they are consuming food. The dopamine-triggered behavioral state of exploitation in nematodes—in which they slow down and search their surroundings for food—is in many ways the most primitive version of wanting. As early as the first bilaterians, dopamine was a signal for the anticipation of a future good thing, not the signal for the good thing itself.
Dopamine and serotonin are primarily involved in navigating the happy side of affective states—the different flavors of positive affect. There are additional neuromodulators, equally ancient, that undergird the mechanisms of negative affect—of stress, anxiety, and depression.
When Worms Get Stressed
These people aren’t being eaten by lions, or starving, or freezing to death. These people are dying because their brains are killing them. Choosing to commit suicide, knowingly consuming deadly drugs, or binge eating oneself into obesity are, of course, behaviors generated by our brains. Any attempt to understand animal behavior, brains, and intelligence itself is wholly incomplete without understanding this enigma: Why would evolution have created brains with such a catastrophic and seemingly ridiculous flaw? The point of brains, as with all evolutionary adaptations, is to improve survival. Why, then, do brains generate such obviously self-destructive behaviors?
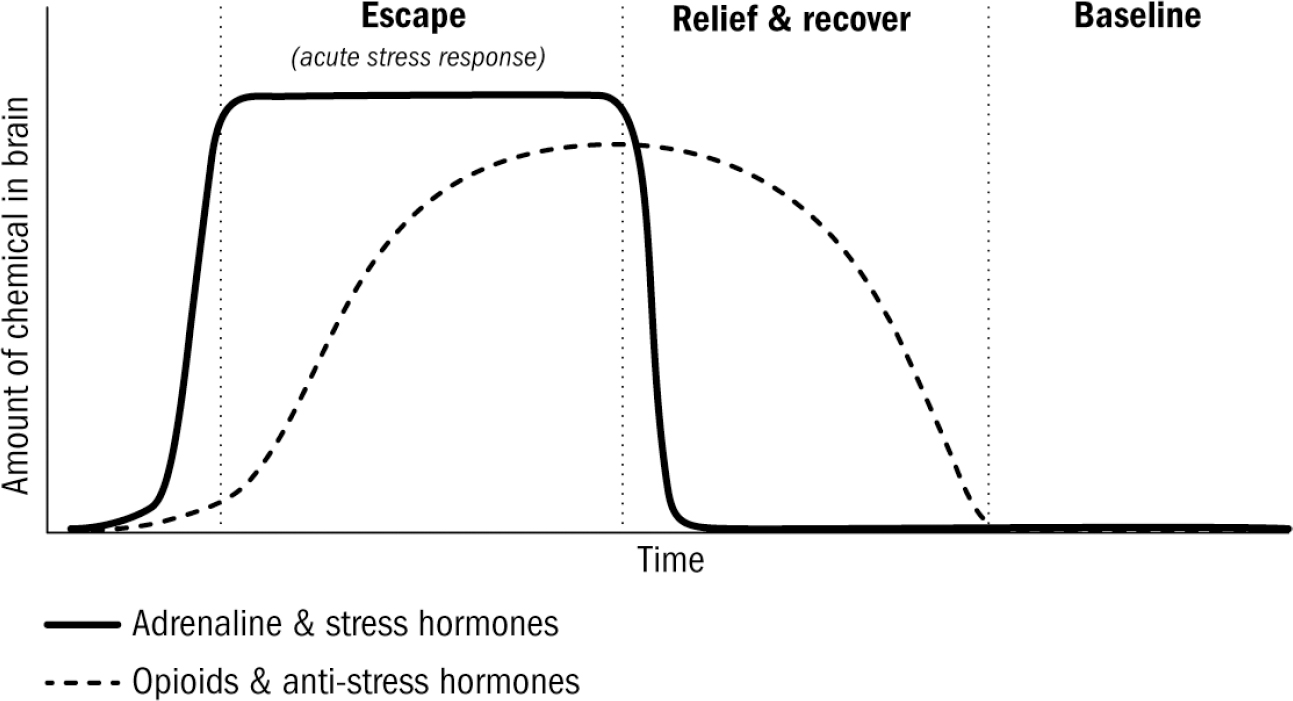
Figure 3.6: The time course of stress and anti-stress hormones
Original art by Rebecca Gelernter
These anti-stress hormones like opioids differed from dopamine and serotonin in Kent Berridge’s rat facial expression experiments. While dopamine had no impact on liking reactions, giving opioids to a rat did, in fact, substantially increase their liking reactions to food. This makes sense given what we now know about the evolutionary origin of opioids. Opioids are the relief-and-recover chemical after experiencing stress: stress hormones turn positive-valence responses off (decreasing liking), but when a stressor is gone, the leftover opioids turn these valence responses back on (increasing liking). Opioids make everything better; they increase liking reactions and decrease disliking reactions; increasing pleasure and inhibiting pain.
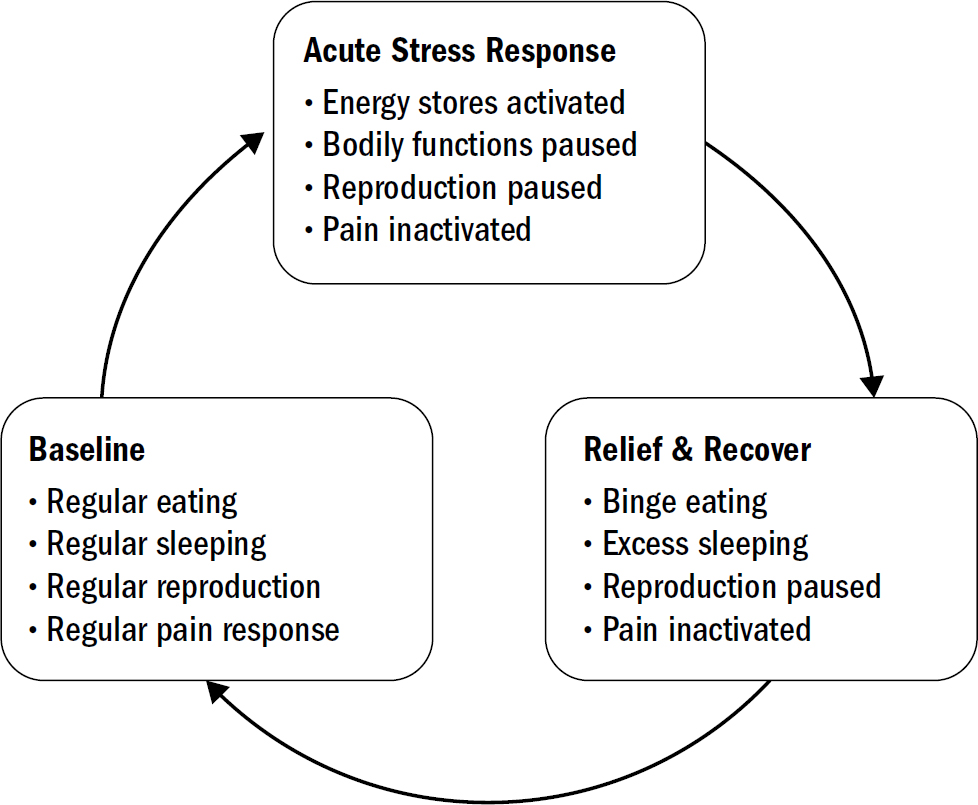
Figure 3.7: The ancient stress cycle, originating from first bilaterians
Original art by Rebecca Gelernter
The Blahs and Blues
We have invented drugs that hack these ancient systems. The euphoria provided by natural opioids is meant to be reserved for that brief period after a near-death experience. But humans can now indiscriminately trigger this state with nothing more than a pill. This creates a problem. Repeatedly flooding the brain with opioids creates a state of chronic stress when the drug wears off—adaptation is unavoidable. This then traps opioid users in a vicious cycle of relief, adaptation, chronic stress requiring more drugs to get back to baseline, which causes more adaptation and thereby more chronic stress. Evolutionary constraints cast a long shadow on modern humanity.
These primitive affective states were passed down and elaborated throughout evolution, and remnants are still—whether we like it or not—essential cornerstones of human behavior. Over time, neuromodulators were repurposed for different functions, and new variants of each of these affective states emerged. And so, while the modern emotional states of humans are undeniably more complex and nuanced than a simple two-by-two grid of valence and arousal, they nonetheless retain the scaffolding of the basic template from which they evolved.
This leaves us at the doorstep of a surprising hypothesis: Affect, despite all its modern color, evolved 550 million years ago in early bilaterians for nothing more than the mundane purpose of steering. The basic template of affect seems to have emerged from two fundamental questions in steering. The first was the arousal question: Do I want to expend energy moving or not? The second was the valence question: Do I want to stay in this location or leave this location? The release of specific neuromodulators enforced specific answers to each of these questions. And these global signals for stay and leave could then be used to modulate suites of reflexes, such as whether it was safe to lay eggs, mate, and expend energy digesting food.
However, these affective states and their neuromodulators would go on to play an even more foundational role in the evolution of the first brains.
4
Associating, Predicting, and the Dawn of Learning
Memory is everything. Without
—ERIC KANDEL
ON DECEMBER 12, 1904, a Russian scientist by the name of Ivan Pavlov stood in front of an assembly of researchers at the Karolinska Institute in Sweden. Pavlov had, two days earlier, become the first Russian to win the Nobel Prize. Eight years prior, Alfred Nobel—the Swedish engineer and businessman who got rich from his invention of dynamite—had passed away and bequeathed his fortune to the creation of the Nobel Foundation. Nobel had stipulated that winners were to give a lecture on the subject for which the prize had been awarded, and so, on this day in Stockholm, Pavlov gave his lecture.
Although he is currently known for his contributions to psychology, that was not the work that earned him the Nobel. Pavlov was not a psychologist but a physiologist—he had spent his entire research career up to this point studying the underlying biological mechanisms—the “physiology”—of the digestive system.
Before Pavlov, the only way to study the digestive system was to surgically remove animals’ organs—esophagus, stomach, or pancreas—and run experiments quickly before the organs died. Pavlov pioneered a variety of relatively noninvasive techniques that enabled him to measure features of the digestive system in intact and healthy dogs. The most famous of these was the insertion of a small salivary fistula that diverted saliva from one salivary gland to a small tube that hung out of the dog’s mouth; this enabled Pavlov to determine the quantity and content of saliva produced by various stimuli. He did similar tricks with the esophagus, stomach, and pancreas.
Through these new techniques, Pavlov and his colleagues made several discoveries. They learned what types of digestive chemicals were released in response to various foods, and he discovered that the digestive organs were under the control of the nervous system. These contributions won him the prize.
However, two-thirds through his speech, Pavlov turned his focus away from his prizewinning work. An excitable scientist, he couldn’t resist pitching research that was, at the time, speculative but that he believed would eventually become his most important work—his exploration of what he called conditional reflexes.
There had always been a pesky confound that got in the way of his meticulous measurements of digestive responses—digestive organs often became stimulated before animals tasted food. His dogs salivated and their stomachs gurgled the moment they realized an experiment with food was about to begin. This was a problem. If you want to measure how salivary glands respond when taste buds detect fatty meat or sugary fruit, you don’t want the confounding measurement of whatever was released by the subjects merely looking at these substances.
Only much later, after bringing psychologists into his lab, did Pavlov begin to view psychic stimulation not as a confound to be eliminated but as a variable worthy of analysis. Ironically, it was a digestive physiologist with the goal of eliminating psychic stimulation who became the first to understand it.
Pavlov’s lab discovered that psychic stimulation was not as random as it seemed. Dogs would salivate in response to any stimuli—metronomes, lights, buzzers—that had been previously associated with food. If an experimenter turned on a buzzer and then gave food, the dog began to salivate in response to the buzzer alone. The dog had developed a conditional reflex—the reflex to salivate in response to the buzzer was conditional on the prior association between the buzzer and food. Pavlov contrasted these conditional reflexes with what he called unconditional reflexes—those that were innate and required no association. A hungry dog’s reflex to salivate in response to sugar placed in its mouth occurred regardless of any prior associations.
If associative learning is a property of simple circuits of neurons, even those present outside of the brain, then it might be a very ancient evolutionary trick. Indeed, Pavlov had unintentionally stumbled on the evolutionary origin of learning itself.
Tweaking the Goodness and Badness of Things
Suppose you took a hundred nematodes, put half of them in a dish with plain water and the other half in a dish with salty water. After several hours, these nematodes will become uncomfortably hungry, as neither dish contains any food. At this point, put both groups of nematodes into another dish that contains a little morsel of salt on one side. What happens?
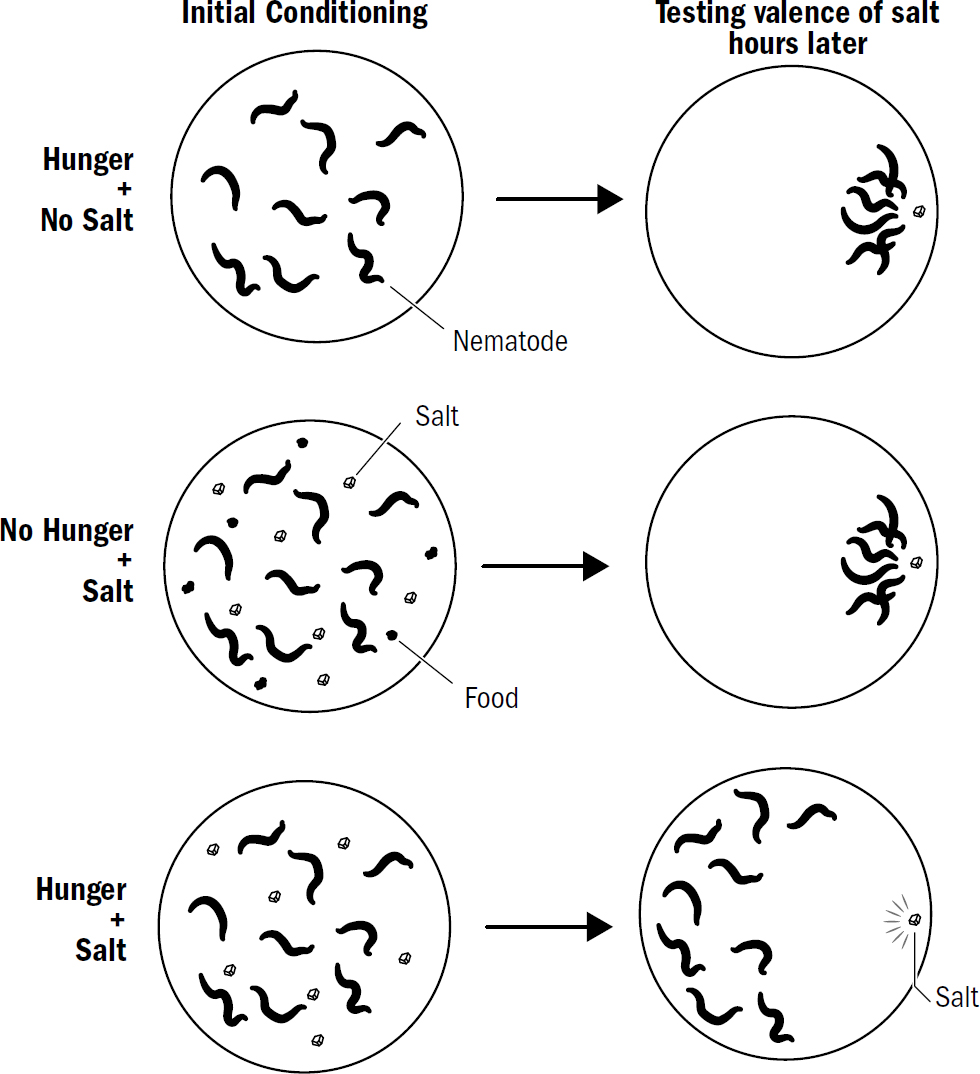
Figure 4.1: Nematodes learn to steer away from salt when salt is associated with hunger
Figure designed by Max Bennett (with some icons from Rebecca Gelernter)
It turns out that Pavlov’s associative learning is an intellectual ability of all bilaterians, even simple ones. If you expose nematodes simultaneously to both a yummy food smell and a noxious chemical that makes them sick, nematodes will subsequently steer away
Why have non-bilaterian animals like coral and anemones, despite an additional six hundred million years of evolution, not acquired the ability to learn associations? Their survival strategy simply doesn’t require it.
A coral polyp with the ability of associative learning wouldn’t survive much better than one without associative learning. A coral polyp just sits in place, immobilized, waiting for food to swim into its tentacles. The hardcoded strategy of swallowing anything that touches its tentacles and withdrawing from anything painful works just fine, without any associative learning. In contrast, a brain designed for steering would have faced unique evolutionary pressure to adjust its steering decisions based on experience. An early bilaterian that could remember to avoid a chemical that had previously been found near predators would survive far better than a bilaterian that could not.
Once animals began approaching specific things and avoiding others, the ability to tweak what was considered good and bad became a matter of life and death.
The Continual Learning Problem
Your self-driving car doesn’t automatically get better as you drive; the facial-recognition technology in your phone doesn’t automatically get better each time you open your phone. As of 2023, most modern AI systems go through a process of training, and once trained, they are sent off into the world to be used, but they no longer learn. This has always presented a problem for AI systems—if the contingencies in the world change in a way not captured in the training data, then these AI systems need to be retrained, otherwise they will make catastrophic mistakes. If new legislation required people to drive on the left side of the road, and AI systems were trained to drive only on the right side of the road, they would not be able to flexibly adjust to the new environment without being explicitly retrained.
While learning in modern AI systems is not continual, learning in biological brains has always been continual. Even our ancestral nematode had no choice but to learn continually. The associations between things were always changing. In some environments, salt was found on food; in others, it was found on barren rocks without food. In some environments, food grew at cool temperatures; in others, it grew at warm temperatures. In some environments, food was found in bright areas; in others, predators were found in bright areas. The first brains needed a mechanism to not only acquire associations but also quickly change these associations to match the changing rules of the world. It was Pavlov who first found hints of these ancient mechanisms.
By measuring the quantity of saliva produced in response to cues that had been paired with food, Pavlov was able to not only observe the presence of associations, but also quantitatively measure the strength of these associations—the more saliva released in response to a cue, the stronger the association. Pavlov had found a way to measure memory. And by recording how memory changed over time, Pavlov could observe the process of continual learning.
Indeed, the associations in Pavlov’s conditional reflexes are always strengthening or weakening with each new experience. In Pavlov’s experiments, the associations strengthened with each subsequent pairing—each time the buzzer occurred before food was given, the more the dog salivated the next time the buzzer occurred. This process is called acquisition (the association was being acquired).
If after learning this association, the buzzer is presented in the absence of food, then the strength of the association fades with each trial,
There are two interesting features of extinction. Suppose you break a previously learned association—sound the buzzer several times in a row, but don’t give food. As expected, dogs will eventually stop salivating at the buzzer. However, if you wait a few days and then sound the buzzer again, something odd happens: dogs start salivating in response to the buzzer again. This is called spontaneous recovery: broken associations are rapidly suppressed but not, in fact, unlearned; given enough time, they reemerge. Further, if after a long stretch of trials with a broken association (buzzer but no food), you reinstate the association (sound a buzzer and provide food again), the old association will be relearned far more rapidly than the first time the dog experienced the association between the buzzer and food. This is called reacquisition: old extinguished associations are reacquired faster than entirely new associations.
Why do associations show spontaneous recovery and reacquisition? Consider the ancient environment in which associative learning evolved. Suppose a worm has many experiences of finding food alongside salt. And then one day, it detects salt, steers toward it, and finds no food. After the worm spends an hour sniffing around without finding food, the association becomes extinguished, and the worm begins steering toward other cues, no longer attracted to salt. If two days later it detects salt again, would it be smarter to steer toward or away from it? In all of the worm’s past experiences, except the most recent one, when it smelled salt it also found food. And so the smarter choice would be to steer toward salt again—the most recent experience may have been a fluke. This is the benefit of spontaneous recovery—it enables a primitive form of long-term memory to persist through the tumult of short-term changes in the contingencies of the world. Of course, if the next twenty times the worm detects salt it fails to find food, the association may eventually be permanently extinguished.
Figure 4.2: The time course of associative learning
Original art by Rebecca Gelernter
The effect of reacqusition—the accelerated relearning of old previously broken associations—evolved in ancient worms for similar reasons. Suppose this same worm finds salt alongside food after the association was long ago extinguished. How quickly should the worm restrengthen the association between salt and food? It would make sense to relearn this association rapidly, given the long-term memory the worm has: In some cases, salt leads to food, and it seems that right now is one of those situations! Thus, old associations are primed to reemerge whenever the world provides hints that old contingencies are newly reestablished.
Spontaneous recovery and reacquisition enabled simple steering brains to navigate changing associations, temporarily suppress old associations that were currently inaccurate, and remember and relearn broken associations that became effective again.
The Credit Assignment Problem
Associative learning comes with another problem: When an animal gets food, there is never a single predictive cue beforehand but rather a whole swath of cues. If you pair a tap to the side of a slug with a shock, how does a slug’s brain know to associate only the tap with the shock and not the many other sensory stimuli that were present, such as the surrounding temperature, the texture of the ground, or the diverse chemicals floating around the seawater? In machine learning, this is called the credit assignment problem: When something happens, what previous cue do you give credit for predicting it? The ancient bilaterian brain, which was capable of only the simplest forms of learning, employed four tricks to solve the credit assignment problem. These tricks were both crude and clever, and they became foundational mechanisms for how neurons make associations in all their bilaterian descendants.
The first trick used what are called eligibility traces. A slug will associate a tap with a subsequent shock only if the tap occurs one second before the shock. If the tap occurs two seconds or more before the shock, no association will be made. A stimulus like a tap creates a short eligibility trace that lasts for about a second. Only within this short time window can associations be made. This is clever, as it invokes a reasonable rule of thumb: stimuli that are useful for predicting things should occur right before the thing you are trying to predict.
The second trick was overshadowing. When animals have multiple predictive cues to use, their brains tend to pick the cues that are the strongest—strong cues overshadow weak cues. If a bright light and a weak odor are both present before an event, the bright light, not the weak odor, will be used as the predictive cue.
The third trick was latent inhibition—stimuli that animals regularly experienced in the past are inhibited from making future associations. In other words, frequent stimuli are flagged as irrelevant background noise. Latent inhibition is a clever way to ask, “What was different this time?” If a slug has experienced the current texture of the ground and the current temperature a thousand times but has never experienced a tap before, then the tap is far more likely to be used as a predictive cue.
The Original Four Tricks for Tackling the Credit Assignment Problem
ELIGIBILITY TRACES | OVERSHADOWING | LATENT INHIBITION | BLOCKING |
Pick the predictive cue that occurred between 0 to 1 second before the event. | Pick the predictive cue that was the strongest. | Pick the predictive cue that you haven’t seen before. | Stick to predictive cues once you have them and ignore others. |
The Ancient Mechanisms of Learning
For thousands of years, two groups of philosophers have been debating the relationship between the brain and the mind. One group, the dualists, like Plato, Aquinas, and Descartes, argue that the mind exists separately from the brain. The entities might interact with each other, but they are distinct; the mind is something beyond the physical. The materialists, like Kanada, Democritus, Epicurus, and Hobbes, argued that whatever the mind is, it is located entirely in the physical structure of the brain. There is nothing beyond the physical. This debate still rages in philosophy departments around the world. If you have made it this far in the book, I will assume you lean on the side of materialism, that you—like me—tend to reject nonphysical explanations for things, even the mind. But by siding with the materialists, we introduce several issues that, at first, are hard to explain physically, the most obvious being learning.
You can read a sentence once and then immediately repeat it out loud. If we stick to a materialist view, this means that reading this sentence instantaneously changed something physical in your brain. Anything that leads to learning causes physical reorganization of something in the 86 billion neurons in each of our heads. Keeping track of a conversation, watching a movie, and learning to tie your shoes all must change the physicality of our brains.
The flurry of discoveries about neurons in the early twentieth century provided a host of new building blocks. The discovery of the connections between neurons—synapses—was the most obvious new thing that could presumably change in the brain during learning. Indeed, it turns out that learning rises not from impressions, folds, or vibrations but from changes to these synaptic connections.
Learning occurs when synapses change their strength or when new synapses are formed or old synapses are removed. If the connection between two neurons is weak, the input neuron will have to fire many spikes to get the output neuron to spike. If the connection is strong, the input neuron will have to fire only a few spikes to get the output neuron to spike. Synapses can increase their strength by the input neuron releasing more neurotransmitter in response to a spike or the postsynaptic neuron increasing the number of protein receptors (hence more responsive to the same quantity of neurotransmitter).
Figure 4.3
Figure by Max Bennett
But the logic of changing synaptic strengths gets more complex than this. There are molecular mechanisms in synapses to measure timing, whereby associations are built only if the input neuron fires right before the output neuron, thereby enabling the trick of eligibility traces. Neuromodulators like serotonin and dopamine can modify the learning rules of synapses; some synapses undergo Hebbian learning only when dopamine or serotonin receptors are also activated, thereby enabling neuromodulators to gate the ability of synapses to build new associations. A worm that sniffs a chemical and then finds food has its brain flooded with dopamine, which then can trigger the strengthening of specific synapses.
Although we don’t yet fully understand all the mechanisms by which neurons rewire themselves, these mechanisms are remarkably similar among bilaterians; the neurons in the brain of a nematode change their synapses in largely the same way as the neurons in your brain. In contrast, when we examine the neurons and synapses of non-bilaterians like coral polyps, we do not find the same machinery; for example, they lack certain proteins known to be
Learning had humble beginnings. While early bilaterians were the first to learn associations, they were still unable to learn most things. They could not learn to associate events separated by more than a few seconds; they could not learn to predict the exact timing of things; they could not learn to recognize objects; they could not recognize patterns in the world; and they could not learn to recognize locations or directions.
But still, the ability of the human brain to rewire itself, to make associations between things, is not a uniquely human superpower but one we inherited from this ancient bilaterian ancestor that lived over 550 million years ago. All the feats of learning that followed (the ability to learn spatial maps, language, object recognition, music, and everything else) were built on these same learning mechanisms. From the bilaterian brain onward, the evolution of learning was primarily a process of finding new applications of preexisting synaptic learning mechanisms, without changing the learning mechanisms themselves.
Learning was not the core function of the first brain; it was merely a feature, a trick to optimize steering decisions. Association, prediction, and learning emerged for tweaking the goodness and badness of things. In some sense, the evolutionary story that will follow is one of learning being transformed from a cute feature of the brain to its core function. Indeed, the next breakthrough in brain evolution was all about a brilliant new form of learning, one that was possible only because it was built on the foundation of valence, affect, and associative learning.
Summary of Breakthrough #1: Steering
Our ancestors from around 550 million years ago transitioned from a radially symmetric brainless animal, like a coral polyp, to a bilaterally symmetric brain-enabled animal, like a nematode. And while many neurological changes occurred across this transition, a surprisingly broad set of them can be understood through the lens of enabling a singular breakthrough: that of navigating by steering. These include:
- A bilateral body plan that reduces navigational choices to two simple options: go forward or turn
- A neural architecture for valence in which stimuli is evolutionarily hard-coded into good and bad
- Mechanisms for modulating valence responses based on internal states
- Circuits whereby different valence neurons can be integrated into a singular steering decision (hence a big cluster of neurons we identify as a brain)
- Affective states for making persistent decisions as to whether to leave or stay
- The stress response for energy management of movements in the presence of hardship
- Associative learning for changing steering decisions based on previous experience
- Spontaneous recovery and reacquisition for dealing with changing contingencies in the world (making continual learning work, even if imperfectly)
- Eligibility traces, overshadowing, latent inhibition, and blocking for (imperfectly) tackling the credit assignment problem
All of these changes made steering possible and solidified our ancestors’ place as the first large multicellular animals who survived by navigating—moving not with microscopic cellular propellers but with muscles and neurons. And all these changes, along with the predatory ecosystem they begot, laid the foundation for breakthrough #2, which was when learning finally took its central role in the function of our brains.
Breakthrough #2
Reinforcing and the First Vertebrates
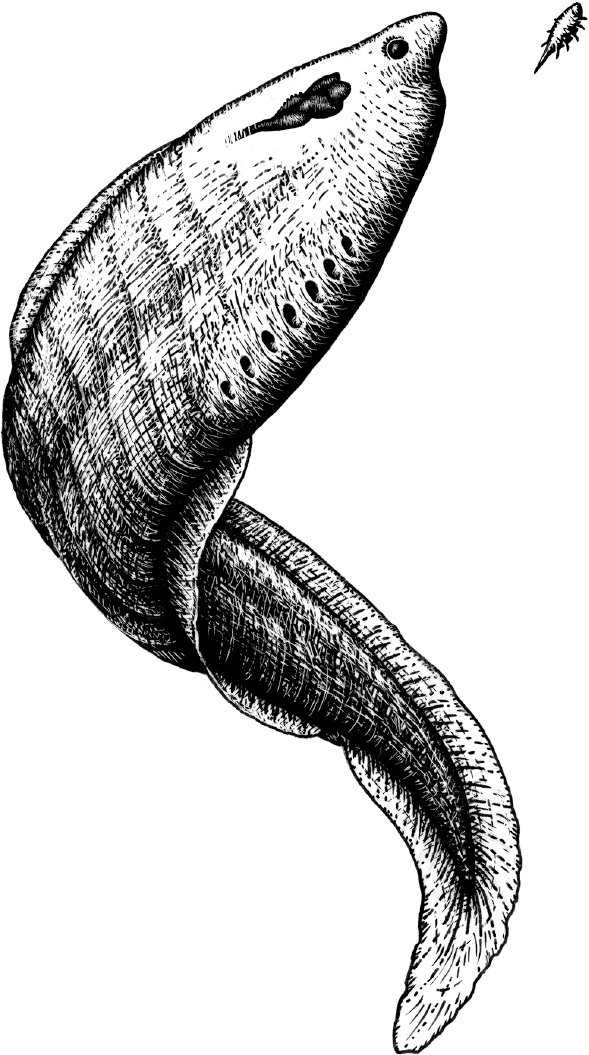
Your brain 500 million years ago
Original art by Rebecca Gelernter
5
The Cambrian Explosion
TO GET TO the next milestone in brain evolution, we must leave the era when the first bilaterians were wiggling around and jump forward fifty million years. The ancient world this brings us to is the Cambrian period, an era that stretched from 540 to 485 million years ago.
If you peered around the Cambrian, you would see a world very different from the older Ediacaran. The gooey microbial mats of the Ediacaran that turned the ocean floor green would have long since faded and given way to a more familiar sandy underbelly. The sensile, slow, and small creatures of the Ediacaran would have been replaced by a bustling zoo of large mobile animals as varied in form as in size. This wouldn’t resemble a zoo you would enjoy—this was a world ruled by arthropods, the ancestors of insects, spiders, and crustaceans. These arthropods were far more terrifying than their modern descendants; they were massive and armed with hauntingly oversize claws and armored shells. Some grew to over five feet long.
The discovery of steering in our nematode-like ancestor accelerated the evolutionary arms race of predation. This triggered what is now known as the Cambrian explosion, the most dramatic expansion in the diversity of animal life Earth has ever seen. Ediacaran fossils are rare and sought after, but Cambrian fossils, if you dig deep enough, are all over the place, and they encompass a mind-boggling diversity of creatures. During the Ediacaran period, animals with brains were humble inhabitants of the seafloor, smaller and less numerous than their brainless animal cousins like the coral and anemones. During the Cambrian period, however, animals with brains began their reign over the animal kingdom.
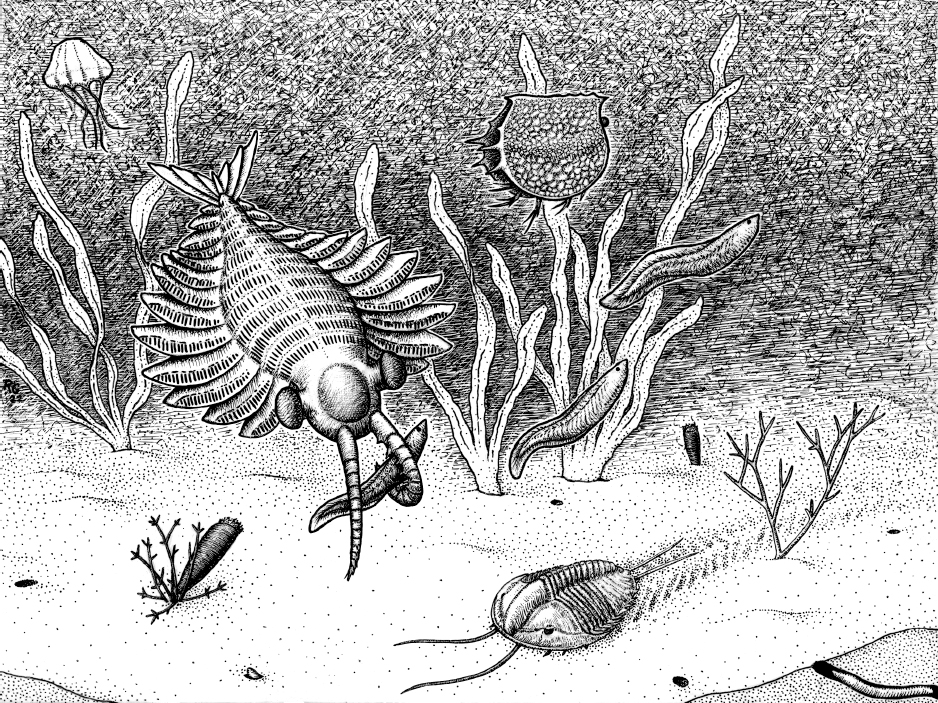
Figure 5.1: The Cambrian world
Original art by Rebecca Gelernter
One lineage of Grandma Worm remained relatively unchanged and shrank in size, becoming the nematodes of today. Another lineage became the masters of this era, the arthropods. Lineages of these arthropods would independently develop their own brain structures with their own intellectual abilities. Some, such as the ants and honeybees, would go on to become impressively smart. But neither the arthropod nor the nematode lineage is ours. Our ancestors were likely not very conspicuous in the Cambrian cacophony of terrifying creatures; they were barely bigger than early bilaterians, only a few inches long, and not particularly numerous. But if you spotted them, they would have looked refreshingly familiar—they would have resembled a modern fish.
Fossil records of these ancient fish show several familiar features. They had fins, gills, a spinal cord, two eyes, nostrils, and a heart. The easiest-to-spot feature in fossils of these creatures is the vertebral column, the thick interlocking bones that encased and protected their spinal cord. Indeed, taxonomists refer to the descendants of this ancient fishlike ancestor as vertebrates. But of all the familiar changes that emerged in these early vertebrates, the most remarkable was surely the brain.
The Vertebrate Brain Template
The brains of invertebrates (nematodes, ants, bees, earthworms) have no recognizably similar structures to the brains of humans. The evolutionary distance between humans and invertebrates is too distant; our brains are derived from too basic a template in our bilaterian ancestor to reveal any common structures. But when we peer into the brain of even the most distant vertebrates, such as the jawless lamprey fish—with whom our most recent common ancestor was the first vertebrate over five hundred million years ago—we see a brain that shares not only some of the same structures but most of them.
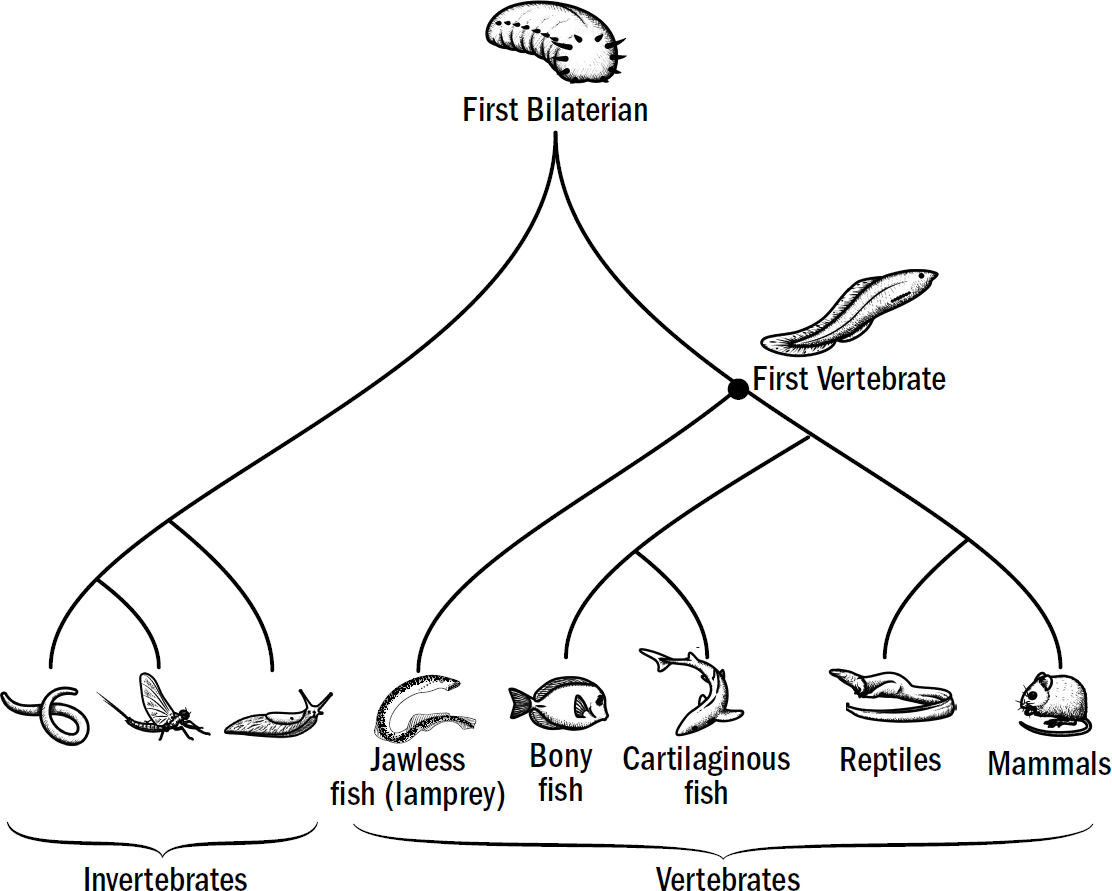
Figure 5.2: Our Cambrian ancestors
Original art by Rebecca Gelernter
From the heat of the Cambrian explosion was forged the vertebrate brain template, one that, even today, is shared across all the descendants of these early fishlike creatures. If you want a crash course in how the human brain works, learning how the fish brain works will get you half of the way there.
The brains of all vertebrate embryos, from fish to humans, develop in the same initial steps. First, brains differentiate into three bulbs, making up the three primary structures that scaffold all vertebrate brains: a forebrain, midbrain, and hindbrain. Second, the forebrain unfolds into two subsystems. One of these goes on to become the cortex and the basal ganglia, and the other goes on to become the thalamus and the hypothalamus.
This results in the six main structures found in all vertebrate brains: the cortex, basal ganglia, thalamus, hypothalamus, midbrain, and hindbrain. Revealing their common ancestry, these structures are remarkably similar across modern vertebrates (except for the cortex, which has unique modifications in some vertebrates, such as mammals; stay tuned for breakthrough #3). The circuitry of the human basal ganglia, thalamus, hypothalamus, midbrain, and hindbrain and that of a fish
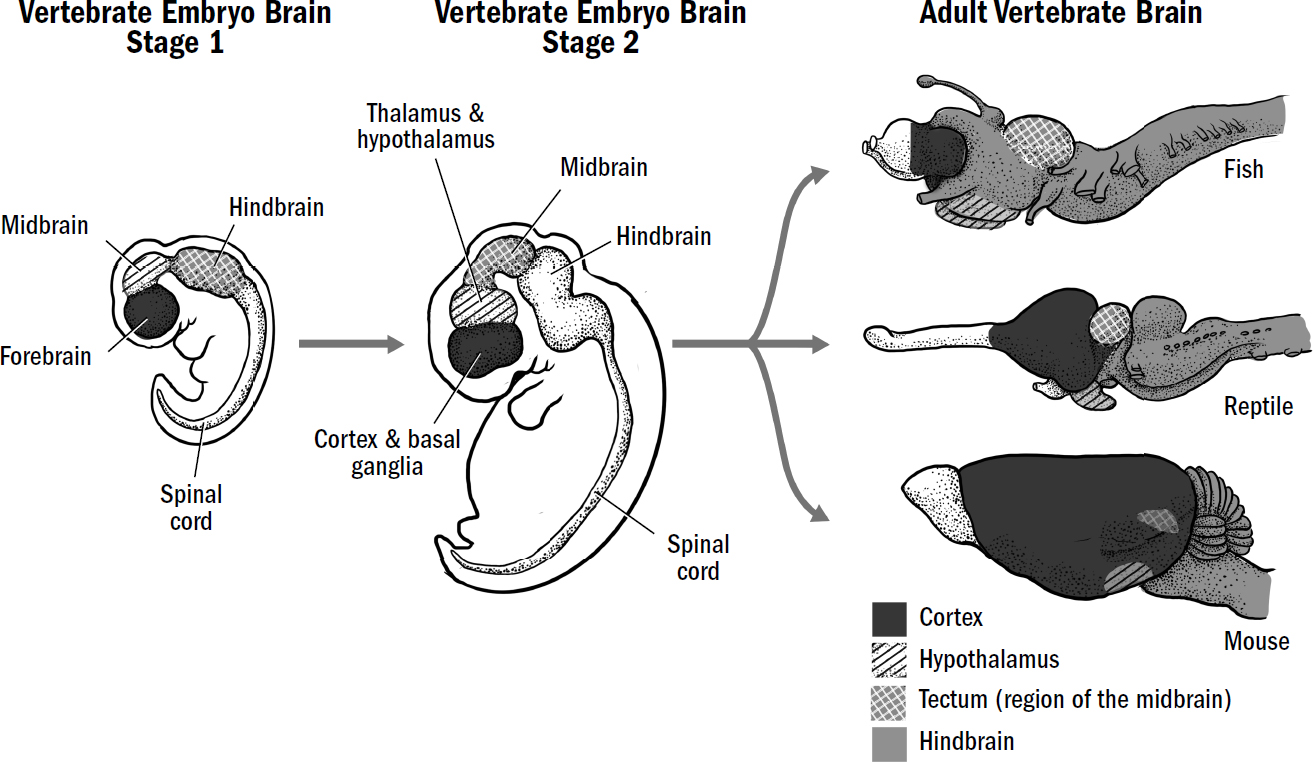
Figure 5.3: The shared embryonic development of vertebrates
Original art by Mesa Schumacher
The first animals gifted us neurons. Then early bilaterians gifted us brains, clustering these neurons into centralized circuits, wiring up the first system for valence, affect, and association. But it was early vertebrates who transformed this simple proto-brain of early bilaterians into a true machine, one with subunits, layers, and processing systems.
The question is, of course, what did this early vertebrate brain do?
Thorndike’s Chickens
Around the same time that Ivan Pavlov was unraveling the inner workings of conditional reflexes in Russia, an American psychologist by the name of Edward Thorndike was probing animal learning from a different perspective.
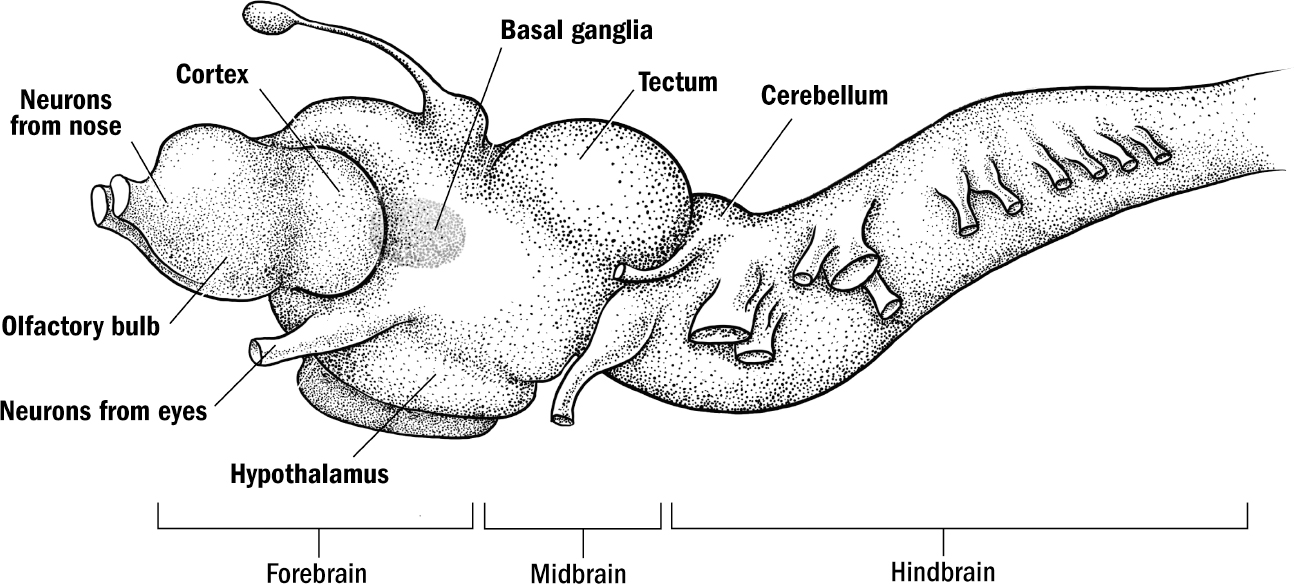
Figure 5.4: The brain of the first vertebrates
Original art by Mesa Schumacher
In 1896, Edward Thorndike found himself in a room full of chickens. Thorndike had recently enrolled in Harvard’s master’s program in psychology. His main interest was studying how children learn: How best can we teach children new things? He had numerous ideas for experiments, but to Thorndike’s chagrin, Harvard would not allow him to conduct experiments on human children. So Thorndike had no choice but to focus on subjects that were easier to obtain: chickens, cats, and dogs.
To Thorndike, this wasn’t all bad. A staunch Darwinist, he was unwavering in his view that there should be common principles in the learning of chickens, cats, dogs, and humans. If these animals shared a common ancestor, then they all should have inherited similar learning mechanisms. By probing how these other animals learned, he believed he might be able to also illuminate the principles of how humans learned.
Thorndike was both extremely shy and incredibly smart, so he was perhaps the perfect person to engage in the solitary, meticulously repetitive, and undeniably clever animal studies that he pioneered. Pavlov did his groundbreaking psychology work when he was middle-aged, after an already famed career as a physiologist, but Thorndike’s most famous work was his first. It was his doctoral dissertation, published in 1898, when he was twenty-three, for which he is most well known. His dissertation: “Animal Intelligence: An Experimental Study of the Associative Processes in Animals.”
Thorndike’s genius, like Pavlov’s, was in how he reduced hopelessly complex theoretical problems to simple measurable experiments. Pavlov explored learning by measuring the amount of saliva released in response to a buzzer. Thorndike explored learning by measuring the speed with which animals learned to escape from what he called puzzle boxes.
Thorndike constructed a multitude of cages, each with a different puzzle inside that, if solved correctly, would open an escape door. These puzzles weren’t particularly complex—some had latches that when pushed would open the door; others had hidden buttons; others had hoops to pull. Sometimes the puzzle did not require a physical contraption, and Thorndike would just manually open the door whenever the animal did something specific, such as lick itself. He placed various animals in these cages, put food outside to motivate the animals to get out of the boxes, and measured exactly how long it took them to figure out the puzzle.
Once the animal escaped, he would record the animal’s time, and then have the animal do it again, and again, and again. He would calculate the average time it took animals to solve a given puzzle on their first trial, compare that with the time for their second, and go all the way to how fast they solved it after as many as a hundred trials.
Image from Thorndike, 1898
Figure 5.6: Animals learning through trial and error
Images from Thorndike, 1898
When first placed in a cage, the cat would try a whole host of behaviors: scratching at the bars, pushing at the ceiling, digging at the door, howling, trying to squeeze through the bars, pacing around the cage. Eventually the cat would accidently press the button or pull the hoop, and the door would open; the cat would exit and happily eat its prize. The animals became progressively faster at repeating the behaviors that got them out of the box. After many trials, cats stopped doing any of their original behaviors and immediately performed the actions required to escape. These cats were learning through trial and error. He could quantify this trial-and-error learning with the gradual decay in the time it took for animals to escape (f igure 5.6).
What was most surprising was how much intelligent behavior emerged from something as simple as trial-and-error learning. After enough trials, these animals could effortlessly perform incredibly complex sequences of actions. It was originally believed that the only way to explain such intelligent behavior in animals was through some notion of insight or imitation or planning, but Thorndike showed how simple trial and error was all an animal really needed. Thorndike summarized his result in his now famous law of effect:
Animals learn by first performing random exploratory actions and then adjusting future actions based on valence outcomes—positive valence reinforces recently performed actions, and negative valence un-reinforces previously performed actions. The terms satisfying and discomforting went out of favor over the decades following Thorndike’s original research; they had an uncomfortable allusion to an actual internal sensation or feeling. Psychologists, including Thorndike, eventually replaced the terms satisfying and discomforting with reinforcing and punishing.
Thorndike’s original research was on cats, dogs, and birds—animals that share a common ancestor around 350 million years ago. But what about more distant vertebrate cousins, those that we share an ancestor with as far back as 500 million years ago? Do they too learn through trial and error?
A year after his 1898 dissertation, Thorndike published an additional note showing the results of these same studies performed on a different animal: fish.
The Surprising Smarts of Fish
If there is any member of the vertebrate group that humans bear the most prejudice against, it is fish. The idea that fish are, well, dumb is embedded in many cultures. We have all heard the folklore that fish cannot retain memories for more than three seconds. Perhaps all this prejudice is to be expected; fish are the vertebrates that are the least like us. But this prejudice is unfounded; fish are far smarter than we give them credit for.
In Thorndike’s original experiment, he put a fish in a tank with a series of transparent walls with hidden openings. He put the fish on one side of the tank (in a bright light, which fish dislike), and on the other side of the tank was a desirable location (the dark, which fish prefer). At first, the fish tried lots of random things to get across the tank, frequently banging into parts of the transparent wall. Eventually the fish found one of the gaps and made it through to the next wall. It then repeated the process until it found the next gap. Once the fish made it past all the walls to the other side, Thorndike picked it up, brought it back to the beginning, and had it start again, each time clocking how long it took the fish to get to the other side. Just as Thorndike’s cats learned to escape puzzle boxes through trial and error, so did his fish learn to quickly zip through each of the hidden openings to escape the bright side of the tank.
This ability of fish to learn arbitrary sequences of actions through trial and error has been replicated many times. Fish can learn to find and push a
If you tried to teach a simple bilaterian like a nematode, flatworm, or slug to perform any of these tasks, it would fail. A nematode cannot be trained to perform arbitrary sequences of actions; it will never learn to navigate through hoops to get food.
Over the next four chapters we will explore the challenges of reinforcement learning and learn why ancestral bilaterians, like modern nematodes, were unable to learn this way. We will learn about how the first vertebrate brains worked, how they overcame these earlier challenges, and how these brains flowered into general reinforcement learning machines.
The second breakthrough was reinforcement learning: the ability to learn arbitrary sequences of actions through trial and error. Thorndike’s idea of trial-and-error learning sounds so simple—reinforce behaviors that lead to good things and punish behaviors that lead to bad things. But this is an example where our intuitions about what is intellectually easy and what is hard are mistaken. It was only when scientists tried to get AI systems to learn through reinforcement that they realized that it wasn’t as easy as Thorndike had thought.
6
The Evolution of Temporal Difference Learning
THE FIRST REINFORCEMENT learning computer algorithm was built in 1951 by a doctoral student at Princeton named Marvin Minsky. This was the beginning of the first wave of excitement around artificial intelligence. In fact, it was Minsky who originally coined the term artificial intelligence. The prior decade had seen the development of the main building blocks for AI: Alan Turing had published his mathematical formulation of general purpose problem-solving machines; the global war effort in the 1940s led to the development of modern computers; an understanding of how neurons worked was beginning to provide clues to how biological brains worked on the micro level; and the study of animal psychology in the vein of Thorndike’s law of effect had provided general principles for how animal intelligence worked on the macro level.
And so Marvin Minsky set out to build an algorithm that would learn like a Thorndikian animal. He named his algorithm the Stochastic Neural-Analog Reinforcement Calculator, or SNARC. He created an artificial neural network with forty connections and trained it to navigate through various mazes. The training process was simple: whenever his system successfully got out of the maze, he strengthened the recently activated synapses. Like Thorndike training a cat to escape a puzzle box with food reinforcements, Minsky was training an AI to escape mazes with numerical reinforcements.
Minsky’s SNARC did not work well. The algorithm got better at navigating out of simple mazes over time, but whenever it faced even slightly more complex situations, it failed. Minsky was one of the first to realize that training algorithms the way that Thorndike believed animals learned—by directly reinforcing positive outcomes and punishing negative outcomes—was
Here’s why. Suppose we teach an AI to play checkers using Thorndike’s version of trial-and-error learning. This AI would start by making random moves, and we would give it a reward whenever it won and a punishment whenever it lost. Presumably, if it played enough games of checkers, it should get better. But here’s the problem: The reinforcements and punishments in a game of checkers—the outcome of winning or losing—occur only at the end of the game. A game can consist of hundreds of moves. If you win, which moves should get credit for being good? If you lose, which moves should get credit for being bad?
One solution is to reinforce or punish actions that occurred recently before winning or losing. The greater the time window between an action and a reward, the less it gets reinforced. This was how Minsky’s SNARC worked. But this works only in situations with short time windows. Even in the game of checkers this is an untenable solution. If a checkers-playing AI assigned credit in this way, then the moves toward the end of the game would always get most of the credit and those toward the beginning very little. This would be dumb—the entire game might have been won on a single clever move in the beginning, long before the game was actually won or lost.
An alternative solution is to reinforce all the prior moves at the end of a winning game (or conversely, punish all the prior moves at the end of a losing game). Your opening blunder, the tide-turning move in the middle, and the inevitable finish will all get reinforced or punished equally depending on whether you won or lost. The argument goes like this: If the AI plays enough games, it will eventually be able to tell the difference between the specific moves that were good and those that were bad.
But this solution also does not work. There are too many configurations of games to learn which moves are good in any reasonable amount of time. There are over five hundred quintillion possible games of checkers. There are over 10120 possible games of chess (more than the number of atoms in the universe). Such a method would require an AI to play so many games that we would all be long dead before it became an even reasonably good player.
This leaves us stuck. When training an AI to play checkers, navigate a maze, or do any other task using reinforcement learning we cannot merely reinforce recent moves and we cannot merely reinforce all the moves. How, then, can AI ever learn through reinforcement?
Minsky identified the temporal credit assignment problem as far back as 1961, but it was left unsolved for decades. The problem was so severe that it rendered reinforcement learning algorithms impotent to solve real-world problems, let alone play a simple game of checkers.
And yet today, artificial reinforcement learning algorithms work far better. Reinforcement learning models are becoming progressively more common in technologies all around us; self-driving cars, personalized ads, and factory robots are frequently powered by them.
How did we get from the complete hopelessness of reinforcement learning in the 1960s to the boom of today?
A Magical Bootstrapping
In 1984, decades after Minsky, a man named Richard Sutton submitted his final PhD dissertation. Sutton proposed a new strategy for solving the temporal credit assignment problem. He had spent the prior six years as a graduate student at UMass Amherst under the supervision of the postdoc Andrew Barto. Sutton and Barto dug up old ideas on reinforcement learning and attempted another stab at it. Six years of work culminated with Sutton’s dissertation, in which he laid one of the intellectual cornerstones for the reinforcement learning revolution. Its title: “Temporal Credit Assignment in Reinforcement Learning.”
Sutton—who had studied psychology, not computer science, as an undergraduate—tackled the problem from a uniquely biological perspective. He didn’t want to understand the best way to tackle the temporal credit assignment problem; he wanted to understand the actual way that animals solved it. Sutton’s undergraduate thesis was titled “A Unified Theory of Expectation.” And Sutton had a hunch that expectation was what was missing from previous attempts to make reinforcement learning work.
Sutton proposed a simple but radical idea. Instead of reinforcing behaviors using actual rewards, what if you reinforced
Imagine you are playing checkers. For the first nine moves, it is mostly neck and neck between you and your opponent. And then on the tenth move you pull off some clever maneuver that turns the tide of the game; suddenly you realize you are in a far better position than your opponent. It is that moment where a temporal difference learning signal reinforces your action.
This, Sutton proposed, might solve the temporal credit assignment problem. This would enable an AI system to learn as it goes instead of having to wait until the end of each game. An AI system can reinforce some moves and punish others throughout a long game of checkers, whether or not it won or lost the overall game. Indeed, sometimes a player makes many good moves in a game he or she ultimately loses, and sometimes a player makes many bad moves in a game he or she ultimately wins.
Figure 6.1
Original art by Rebecca Gelernter
Despite the intuitive appeal of Sutton’s approach, we should not expect it to work. Sutton’s logic is circular. The critic’s prediction of how likely you are to win given a board position depends on what future actions the actor will take (a good board position isn’t good if the actor doesn’t know how to take advantage of it). Similarly, the actor’s decision of what action to take depends on how accurate the critic’s temporal difference reinforcement signals have been at reinforcing and punishing past actions. In other words, the critic depends on the actor, and the actor depends on the critic. This strategy seems doomed from the start.
In his simulations, however, Sutton found that by training an actor and a critic simultaneously, a magical bootstrapping occurs between them. Sure, in the beginning the critic often rewards the wrong actions, and the actor often fails to take the necessary actions to fulfill the predictions of the critic. But over time, with enough games, each refines the other until they converge to produce an AI system capable of making remarkably intelligent decisions. At least, that’s what happened in Sutton’s simulations. It wasn’t clear whether this would work in practice.
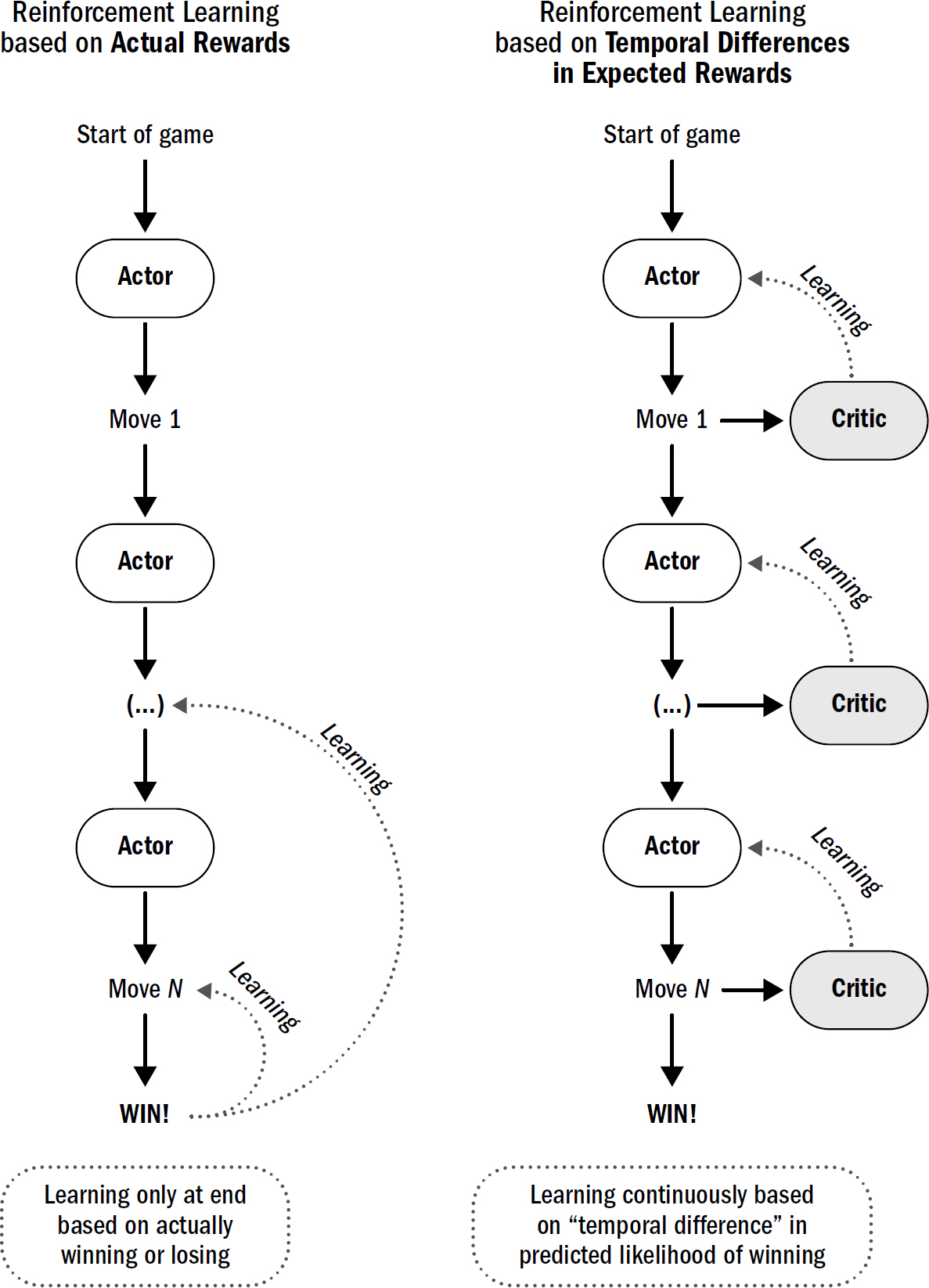
Figure 6.2
Original art by Rebecca Gelernter
At the same time that Sutton was working on TD learning, a young physicist by the name of Gerald Tesauro was working on getting AI systems to play backgammon. Tesauro was at IBM Research, the same group that would later build Deep Blue (the program that famously beat Garry Kasparov in chess) and Watson (the program that famously beat Ken Jennings in Jeopardy!). But before Deep Blue or Watson, there was Neurogammon. Neurogammon was a backgammon-playing AI system that was trained on transcripts of hundreds of expertly played backgammon games. It learned not through trial and error but by attempting to replicate what it believed a human expert would do. By 1989, Neurogammon could beat every other backgammon-playing computer program, but it was lackluster compared to a human, unable to beat even an intermediate-level player.
By the time Tesauro stumbled on Sutton’s work on TD learning, he had spent years trying every conceivable technique to get his computer to play backgammon as well as a human. His crowning achievement was Neurogammon, which was clever but stuck at an intermediate level. And so Tesauro was open to new ideas, even Sutton’s radical idea of allowing a system to teach itself from its own predictions.
It was Tesauro who first put Sutton’s idea to a practical test. In the early 1990s he began working on TD-Gammon, a system that learned to play backgammon using temporal difference learning.
The real question, however, was whether TD learning was merely a clever technique that happened to work or a technique that captured something fundamental about the nature of intelligence. Was TD learning a technological invention, or was it, as Sutton had hoped, an ancient technique that evolution had stumbled upon and long ago weaved into animal brains to make reinforcement learning work?
The Grand Repurposing of Dopamine
While Sutton had hoped there was a connection between his idea and the brain, it was one of his students, Peter Dayan, who found it. At the Salk Institute in San Diego, Dayan and his fellow postdoc Read Montague were convinced that brains implemented some form of TD learning. In the 1990s, emboldened by the success of Tesauro’s TD-Gammon, they went hunting for evidence in the ever-growing mound of neuroscience data.
They knew where to start. Any attempt to understand how reinforcement learning works in vertebrate brains surely had to begin with a little neuromodulator we have already seen: dopamine.
The only way to know what dopamine is signaling is to, well, measure the signal. It wasn’t until the 1980s that technology was advanced enough for scientists to do this. A German neuroscientist named Wolfram Schultz was the first to measure the activity of individual dopamine neurons.
Schultz devised a simple experiment to probe the relationship between dopamine and reinforcement. Schultz showed monkeys different cues (such as pictures of a geometric shape) and then a few seconds later delivered some sugar water into their mouths.
Sure enough, even in this simple reward-prediction task, it was immediately clear that dopamine was not a signal for Thorndike’s satisfying outcomes—it was not a signal for pleasure or valence. At first, dopamine neurons did respond like a valence signal, getting uniquely excited whenever a hungry monkey got sugar water. But after a few trials, dopamine neurons stopped responding to the reward itself and instead responded only to the predictive cue.
When a picture popped up that monkeys knew would lead to sugar, their dopamine neurons got excited, but when these monkeys got sugar water a few moments later, their dopamine neurons did not deviate from their baseline level of activity. Perhaps, then, dopamine was actually a signal for surprise? Perhaps dopamine got excited only when events deviated from expectations, like a surprising picture popping up or a surprsing delivery of sugar water?
When Schultz performed additional experiments, it became clear that this “dopamine as surprise” idea was wrong. Once one of his monkeys had learned to expect sugar water after a specific picture was presented, Schultz again presented this reward-predicting picture but didn’t give sugar. In this case, despite an equal amount of surprise, dopamine activity dramatically declined. While the presentation of an unexpected reward increases dopamine activity, the omission of an expected reward decreases dopamine activity.*
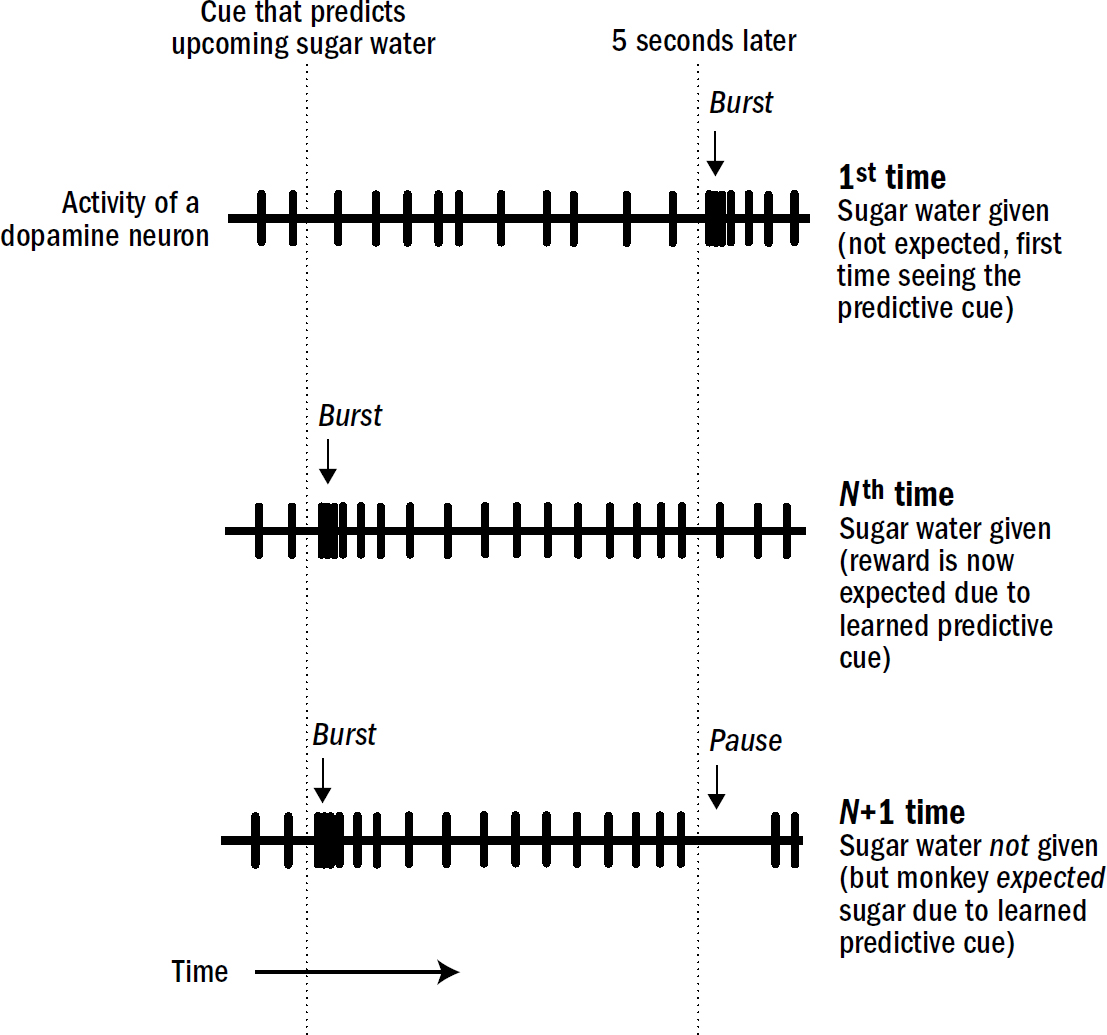
Figure 6.3: Responses of dopamine neurons to predictive cues, rewards, and omissions
Figure by Max Bennett
Schultz was confused by these results. What was dopamine a signal for? If not for valence or pleasure or surprise, then what? Why did dopamine activity shift from rewards to the predictive cues for rewards? Why did dopamine activity decline when expected rewards were omitted?
For many years, the neuroscience community was unsure how to interpret Schultz’s data, an oddity laid bare in the clicks and pauses of an ancient type of neuron.
It wasn’t until a decade later that it was solved. Indeed, it was a decade later when Dayan and Montague began scouring the literature for clues that brains implement some form of TD learning. When they eventually came across Schultz’s data, they immediately knew what they were seeing. The dopamine responses that Schultz found in monkeys aligned exactly with Sutton’s
Even the way dopamine responds to probabilities aligned with a TD-learning signal—a cue that predicts food with a 75 percent probability triggers more dopamine than a cue that predicts food with a 25 percent probability.
Dopamine is not a signal for reward but for reinforcement. As Sutton found, reinforcement and reward must be decoupled for reinforcement learning to work. To solve the temporal credit assignment problem, brains must reinforce behaviors based on changes in predicted future rewards, not actual rewards. This is why animals get addicted to dopamine-releasing behaviors despite it not being pleasurable, and this is why dopamine responses quickly shift their activations to the moments when animals predict upcoming reward and away from rewards themselves.
In early bilaterians, dopamine was a signal for good things nearby—a primitive version of wanting.* In the transition to vertebrates, however, this good-things-are-nearby signal was elaborated to not only trigger a state of wanting but also to communicate a precisely computed temporal difference learning signal. Indeed, it makes sense that dopamine was the neuromodulator that evolution reshaped into a temporal difference learning signal, as the signal for nearby rewards it was the closest thing to a measure of predicted future reward. And so, dopamine was transformed from a good-things-are-nearby signal to a there-is-a-35 percent-chance-of-something-awesome-happening-in-exactly-ten-seconds signal. Repurposed from a fuzzy average of recently detected food to an ever fluctuating, precisely measured, and meticulously computed predicted-future-reward signal.
The Emergence of Relief, Disappointment, and Timing
From the ancient seed of TD learning sprouted several features of intelligence. Two of these—disappointment and relief—are so familiar that they almost disappear from view, so ubiquitous that it is easy to miss the unavoidable fact that they did not always exist. Both disappointment and relief are emergent properties of a brain designed to learn by predicting future rewards. Indeed, without an accurate prediction of a future reward, there can be no disappointment when it does not occur. And without an accurate prediction of future pain, there can be no relief when it does not occur.
Consider the following task of a fish learning through trial and error. If you turn on a light and then after five seconds gently zap the fish if it does not swim to the opposite side of a tank, it will learn to automatically swim to the opposite side of the tank whenever you turn the light on. Seems like straightforward trial-and-error learning right? Unfortunately not. The ability of vertebrates to perform this type of task—called an avoidance task—has long been a source of debate among animal psychologists.
How would Thorndike have explained a fish’s ability to do this? When one of Thorndike’s cats finally got out of a puzzle box, it was the presence of food rewards that reinforced the cat’s actions. But when our fish swam to the safe location, it was the omission of a predicted shock that reinforced the fish’s actions. How can the absence of something be reinforcing?
The answer is that the omission of an expected punishment is itself reinforcing; it is relieving. And the omission of an expected reward is itself punishing; it is disappointing. This is why the activity of Schultz’s dopamine neurons decreased when food was omitted. He was observing the biological manifestation of disappointment—the brain’s punishment signal
In this intellectual divide between vertebrates and invertebrates we find another familiar feature of intelligence, one that also emerges from TD learning and its counterparts of disappointment and relief. If we looked closely at our fish learning to swim to specific locations to avoid a zap, we would observe something remarkable. When the light turns on, the fish does not immediately dash to safety. Instead, it leisurely ignores the light until just before the end of the five-second interval and then rapidly dashes to safety. In this simple task, fish learn not only what to do but when to do it; fish know that the shock occurs precisely
TD learning, disappointment, relief, and the perception of time are all related. The precise perception of time is a necessary ingredient to learn from omission, to know when to trigger disappointment or relief, and thereby to make TD learning work. Without time perception, a brain cannot know whether something was omitted or simply hasn’t happened yet; our fish would know that the light was associated with a zap but not when it should occur. Our fish would cower in fear in the presence of the light long after the risk of the zap had passed, blind to its own safety. It is only with an inner clock that fish can predict the exact moment the zap would occur and thus, if omitted, the exact moment it deserves a relieving dopamine burst.
The Basal Ganglia
My favorite part of the brain is a structure called the basal ganglia.
For most brain structures, the more one learns about them, the less one understands them—simplified frameworks crumble under the weight of messy complexity, the hallmark of biological systems. But the basal ganglia is different. Its inner wiring reveals a mesmerizing and beautiful design, exposing an orderly computation and function. As one might feel awe that evolution could construct an eye, with such symmetry and elegance, one could equally feel awe that evolution could construct the basal ganglia, also endowed with its own symmetry and elegance.
The basal ganglia is wedged between the cortex and the thalamus (see the figure in the first pages of this book). The input to the basal ganglia comes from the cortex, thalamus, and midbrain, enabling the basal ganglia to monitor an animal’s actions and external environment. Information then flows through a labyrinth of substructures within the basal ganglia, branching and merging, transforming and permuting until it reaches the basal ganglia’s output nucleus, which contains thousands to millions of inhibitory neurons that send massive and powerful connections to motor centers in the brainstem. This output nucleus of the basal ganglia is, by default, activated. The motor circuits of the brainstem are constantly being suppressed and gated by the basal ganglia. It is only when specific neurons in the basal ganglia turn off that specific motor circuits in the brainstem are ungated from activation. The basal ganglia is thereby in a perpetual state of gating and ungating specific actions, operating as a global puppeteer of an animal’s behavior.
The functioning of the basal ganglia is essential to our lives. The cononical symptom of Parkinson’s disease is the inability to initiate movement. Patients will sit in a chair for many minutes before they can muster the will to even sit up. This symptom of Parkinson’s disease primarily emerges due to disruption of the basal ganglia, leaving it in a perpetual state of gating all actions, thereby depriving patients of the ability to initiate even the simplest of movements.
What is the computation performed by the basal ganglia? How does it use incoming information about an animal’s actions and external environment to decide which actions to gate (prevent from occuring) and which actions to ungate (allow to occur)?
In addition to receiving information about an animal’s actions and external environment, the basal ganglia also receives input from a cluster of dopamine neurons. Whenever these dopamine neurons get excited, the basal ganglia is rapidly flooded with dopamine; whenever these dopamine neurons are inhibited, the basal ganglia is rapidly starved of dopamine. The synapses within the basal ganglia have different dopamine receptors, each responding in unique ways; these fluctuating levels of dopamine strengthen and weaken specific synapses, modifying how the basal ganglia processes input.
Remarkably, the circuitry of the basal ganglia is practically identical between a human brain and a lamprey fish brain, two species whose shared ancestors were the first vertebrates over 500 million years ago. The various subclusters, the types of neurons, and the overall function seem to be the same. In the brain of early vertebrates emerged the basal ganglia, the biological locus of reinforcement learning.
Reinforcement learning emerged not from the basal ganglia acting alone, but from an ancient interplay between the basal ganglia and another uniquely vertebrate structure called the hypothalamus, which is a small structure at the base of the forebrain.
In vertebrate brains, dopamine release is initially controlled by the hypothalamus. It is the hypothalamus that houses valence neurons inherited from the valence sensory apparatus of ancestral bilaterians. When you are cold, it is your hypothalamus that triggers shivering and that makes you enjoy warmth; just as when you are hot, it is your hypothalamus that triggers sweating and that makes you enjoy the cold. When your body needs calories, it is your hypothalamus that detects hunger signals in your bloodstream and that makes you hungry. The positive valence food-sensitive neurons in early bilaterians functioned just as the positive valence food-sensitive neurons in your hypothalamus do, becoming highly responsive to food when you are hungry and less responsive to food when you are full. This is why you will be salivating over pizza one moment, but then after engorging yourself want absolutely nothing to do with pizza just ten minutes later.
The hypothalamus doesn’t get excited by predictive cues; it gets excited only when it actually gets what it wants—food when hungry, warmth when cold. The hypothalamus is the decider of actual rewards; in our AI-playing-backgammon metaphor, the hypothalamus tells the brain whether it won or lost the game but not how well it is doing as the game is unfolding.
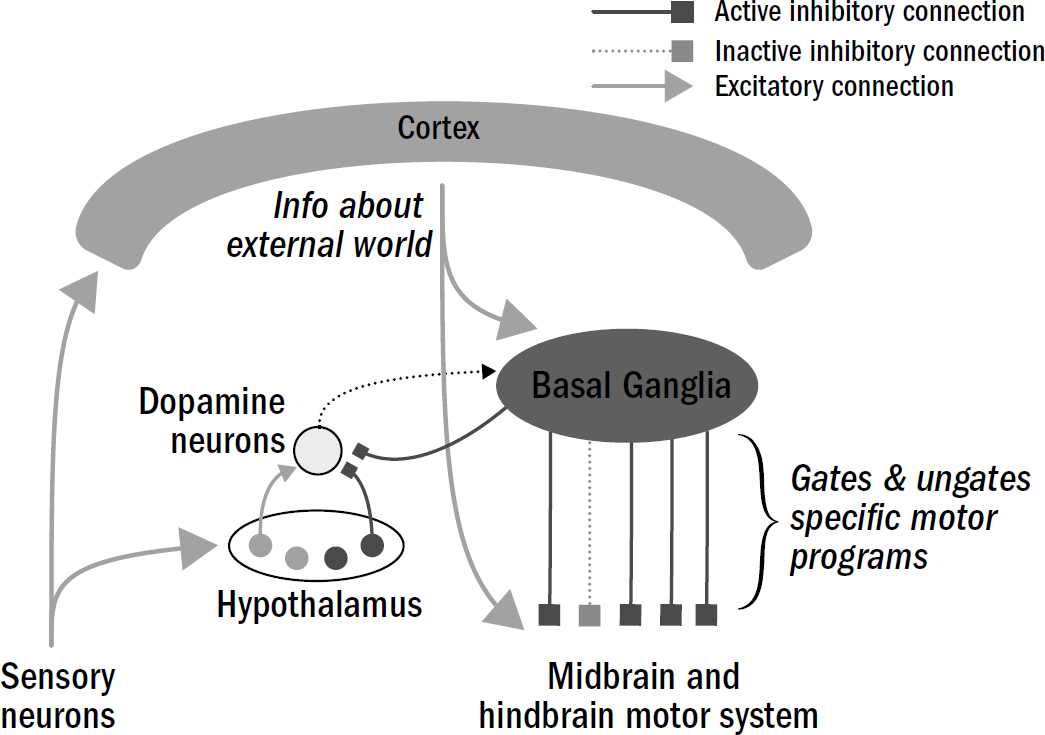
Figure 6.4: A simplified framework for the design of the first vertebrate brain
Original art by Rebecca Gelernter
But as Minsky found with his attempts to make reinforcement learning algorithms in the 1950s, if brains learned only from actual rewards, they would never be able to do anything all that intelligent. They would suffer from the problem of temporal credit assignment. So then how is dopamine transformed from a valence signal for actual rewards to a temporal difference signal for changes in predicted future reward?
In our metaphor, the basal ganglian student initially learns solely from the hypothalamic judge, but over time learns to judge itself, knowing when it makes a mistake before the hypothalamus gives any feedback. This is why dopamine neurons initially respond when rewards are delivered, but over time shift their activation toward predictive cues. This is also why receiving a reward that you knew you were going to receive doesn’t trigger dopamine release; predictions from the basal ganglia cancel out the excitement from the hypothalamus.
The beautifully conserved circuitry of the basal ganglia, first emerging in the minuscule brain of early vertebrates and maintained for five hundred million years, seems to be the biological manifestation of Sutton’s actor-critic system. Sutton discovered a trick that evolution had already stumbled upon over five hundred million years ago.
TD learning, the wiring of vertebrate basal ganglia, the properties of dopamine responses, the ability to learn precise time intervals, and the ability to learn from omissions are all interwoven into the same mechanisms for making trial-and-error learning work.
7
The Problems of Pattern Recognition
FIVE HUNDRED MILLION years ago, the fish-like ancestor of every vertebrate alive today—the inch-long grandmother of every pigeon, shark, mouse, dog, and, yes, human—swam unknowingly toward danger. She swam through the translucent underwater plants of the Cambrian, gently weaving between their thick seaweed-like stalks. She was hunting for coral larvae, the protein-rich offspring of the brainless animals populating the sea. Unbeknownst to her, she too was being hunted.
An Anomalocaris—a foot-long arthropod with two spiked claws sprouting from its head—lay hidden in the sand. Anomalocaris was the apex predator of the Cambrian, and it was waiting patiently for an unlucky creature to come within lunging distance.
Our vertebrate ancestor would have noticed the unfamiliar smell and the irregular-shaped mound of sand in the distance. But there were always unfamiliar smells in the Cambrian ocean; it was a zoo of microbes, plants, fungi, and animals, each releasing their own unique portfolio of scents. And there was always a backdrop of unfamiliar shapes, an ever-moving portrait of countless objects, both living and inanimate. And so she thought nothing of it.
As she emerged from the safety of the Cambrian plants, the arthropod spotted her and lurched forward. Within milliseconds, her reflexive escape response kicked in. Grandma Fish’s eyes detected a fast-moving object in her periphery, triggering a hardwired reflexive turn and dash in the opposite direction. The activation of this escape response flooded her brain with norepinephrine, triggering a state of high arousal, making sensory responses more sensitive, pausing all restorative functions, and reallocating energy to her muscles. In the nick of time, she escaped the clasping talons and swam away.
This has unfolded billions of times, a never-ending cycle of hunting and escaping, of anticipation and fear. But this time was different—our vertebrate ancestor would remember the smell of that dangerous arthropod; she would remember the sight of its eyes peeking through the sand. She wouldn’t make the same mistake again. Sometime around five hundred million years ago, our ancestor evolved pattern recognition.
The Harder-Than-You-Would-Think Problem of Recognizing a Smell
Early bilaterians could not perceive what humans experience as smell. Despite how little effort it takes for you to distinguish the scent of a sunflower from that of a salmon, it is, in fact, a remarkably complicated intellectual feat, one inherited from the first vertebrates.
Just as you have in your nose today, within the nostrils of early vertebrates were thousands of olfactory neurons. In the lamprey fish, there are about fifty different types of olfactory neurons, each type containing a unique olfactory receptor that responds to
Our nematode-like ancestor’s ability to recognize the world was constrained to only the sensory machinery of individual neurons. It could recognize the presence of light by the activation of a single photosensitive neuron or the presence of touch from the activation of a single mechanosensory neuron. Although useful for steering, this rendered a painfully opaque picture of the outside world. Indeed, the brilliance of steering was that it enabled the first bilaterians to find food and avoid predators without perceiving much of anything about the world.
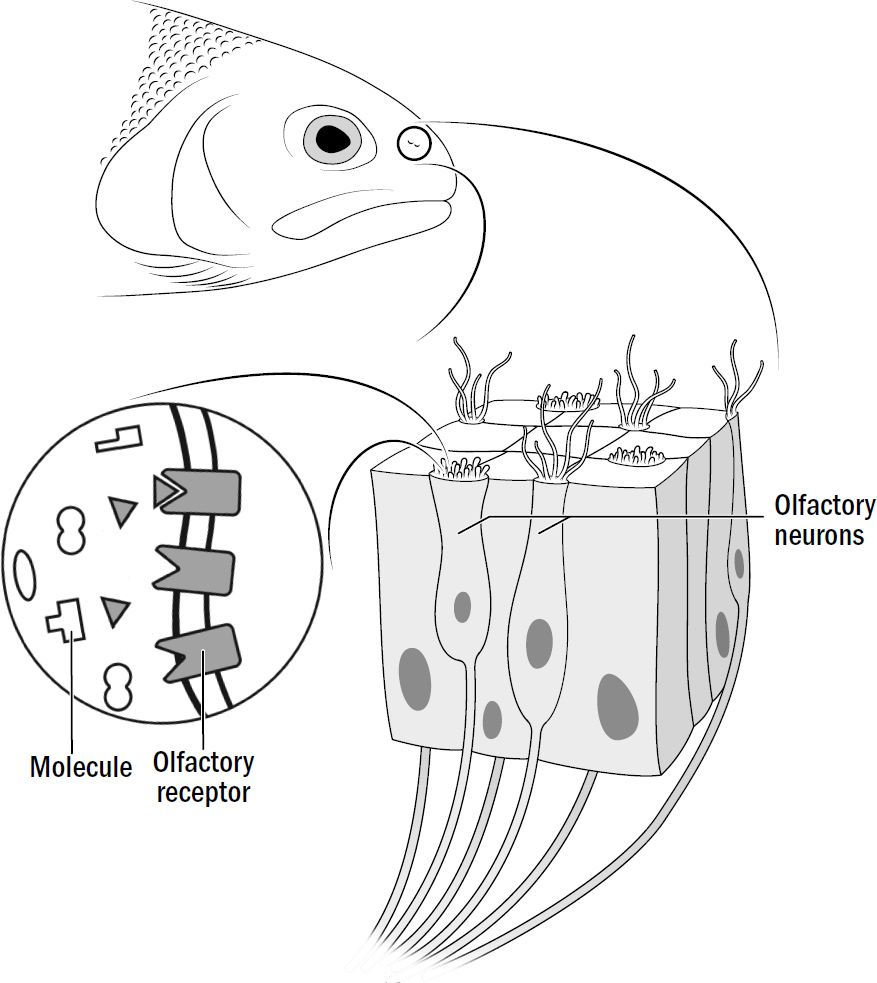
Figure 7.1: Inside the nose of a vertebrate
Original art by Rebecca Gelernter
However, most of the information about the world around you can’t be found in a single activated neuron but only in the pattern of activated neurons. You can distinguish a car from a house based on the pattern of photons hitting your retina. You can distinguish the ramblings of a person from the roar of a panther based on the pattern of sound waves hitting your inner ear. And, yes, you can distinguish the smell of a rose from the smell of chicken based on the pattern of olfactory neurons activated in your nose. For hundreds of millions of years, animals were deprived of this skill, stuck in a perceptual prison.
When you recognize that a plate is too hot or a needle too sharp, you are recognizing attributes of the world the way early bilaterians did, with the activations of individual neurons. However, when you recognize a smell, a face, or a sound, you are recognizing things in the world in a way that was beyond early bilaterians; you are using a skill that emerged later in early vertebrates.
HOW EARLY BILATERIANS RECOGNIZED THINGS IN THE WORLD | HOW EARLY VERTEBRATES RECOGNIZED THINGS IN THE WORLD |
A single neuron detects a specific thing | Brain decodes the pattern of activated neurons to recognize a specific thing |
Small number of things can be recognized | Large number of things can be recognized |
New things can be recognized only through evolutionary tinkering (new sensory machinery needed) | New things can be recognized without evolutionary tinkering but through learning to recognize a new pattern (no new sensory machinery needed) |
Pattern recognition is hard. Many animals alive today, even after another half billion years of evolution, never acquired this ability—the nematodes and flatworms of today show no evidence of pattern recognition.
There were two computational challenges the vertebrate brain needed to solve to recognize patterns. In figure 7.2, you can see an example of three fictional smell patterns: one for a dangerous predator, one for yummy food, and one for an attractive mate. Perhaps you can see from this figure why pattern recognition won’t be easy—these patterns overlap with each other despite having different meanings. One should trigger escape and the others approach. This was the first problem of pattern recognition, that of discrimination: how to recognize overlapping patterns as distinct.
The first time a fish experiences fear in the presence of a novel predator smell, it will remember that specific smell pattern. But the next time the fish encounters that same predator smell, it won’t activate the exact same pattern of olfactory neurons. The balance of molecules will never be identical—the age of the new arthropod, or its sex, or its diet, or many other things might be different that could slightly alter its scent. Even the background smells from the surrounding environment might be different, interfering in slightly different ways. The result of all these minor perturbations is that the next encounter will be similar but not the same. In figure 7.3 you can see three examples of the olfactory patterns that the next encounter with the predator smell might activate. This is the second challenge of pattern recognition: how to generalize a previous pattern to recognize novel patterns that
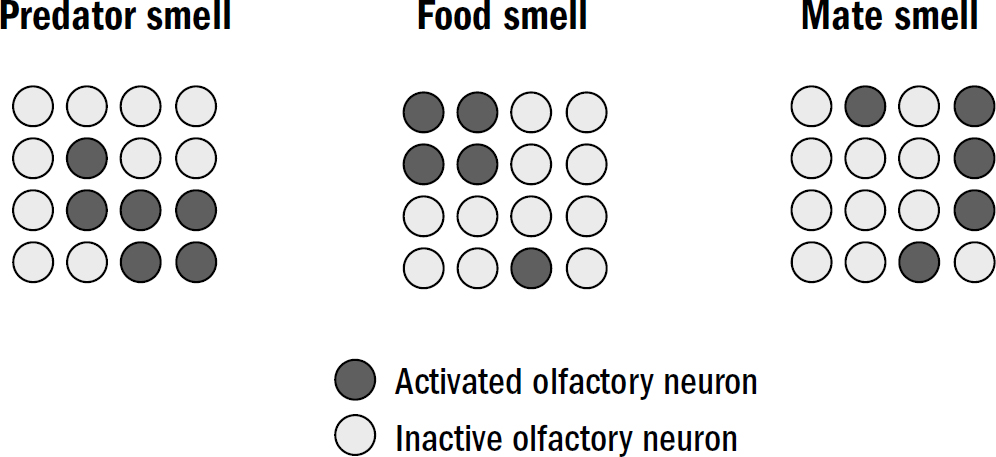
Figure 7.2: The discrimination problem
Figure by Max Bennett
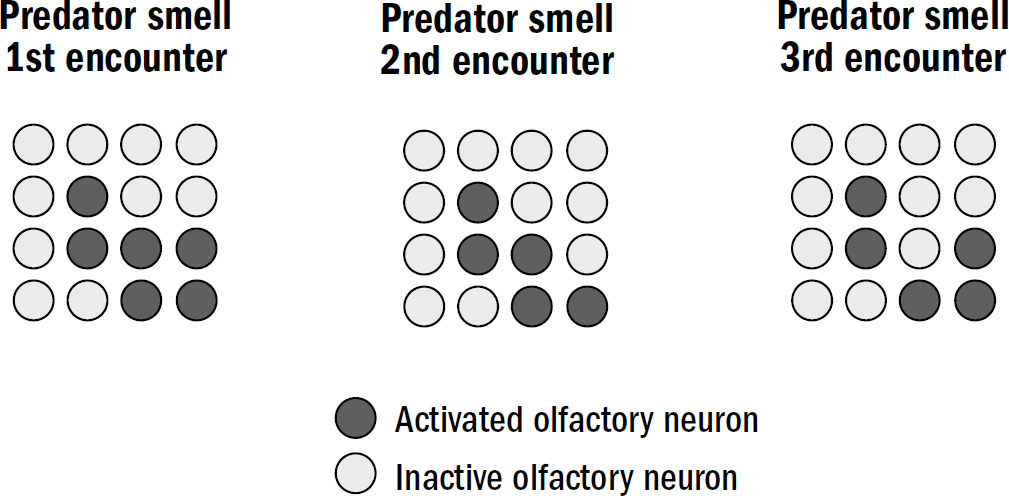
Figure 7.3: The generalization problem
Figure by Max Bennett
How Computers Recognize Patterns
You can unlock your iPhone with your face. Doing this requires your phone to solve the generalization and discrimination problems. Your iPhone needs to be able to tell the difference between your face and other people’s faces, despite the fact that faces have overlapping features (discrimination). And your iPhone needs to identify your face despite changes in shading, angle, facial hair, and more (generalization). Clearly, modern AI systems successfully navigate these two challenges of pattern recognition. How?
Figure 7.4:
Figure by Max Bennett
The hard part is teaching the network how to learn the right weights. The state-of-the-art mechanism for doing this was popularized by Geoffrey Hinton, David Rumelhart, and Ronald Williams in the 1980s. Their method is as follows: If you were training a neural network to categorize smell patterns into egg smells or flower smells, you would show it a bunch of smell patterns and simultaneously tell the network whether each pattern is from an egg or a flower (as measured by the activation of a specific neuron at the end of the network). In other words, you tell the network the correct answer. You then compare the actual output with the desired output and nudge the weights across the entire network in the direction that makes the actual output closer to the desired output. If you do this many times (like, millions of times), the network eventually learns to accurately recognize patterns—it can identify smells of eggs and flowers. They called this learning mechanism backpropagation: they propagate the error at the end back throughout the entire network, calculate the exact error contribution of each synapse, and nudge that synapse accordingly.
The above type of learning, in which a network is trained by providing examples alongside the correct answer, is called supervised learning (a human has supervised the learning process by providing the network with the correct answers). Many supervised learning methods are more complex than this, but the principle is the same: the correct answers are provided, and networks are tweaked using backpropagation to update weights until the categorization of input patterns is sufficiently accurate. This design has proven to work so generally that it is now applied to image recognition, natural language processing, speech recognition, and self-driving cars.
But even one of the inventors of backpropagation, Geoffrey Hinton, realized that his creation, although effective, was a poor model of how the brain actually works. First, the brain does not do supervised learning—you are not given labeled data when you learn that one smell is an egg and another is a strawberry. Even before children learn the words egg and strawberry, they can clearly recognize that they are different. Second, backpropagation is biologically implausible. Backpropagation works by magically nudging millions of synapses simultaneously and in exactly the right amount to move the output of the network in the right direction. There is no conceivable way the brain could do this. So then how does the brain recognize patterns?
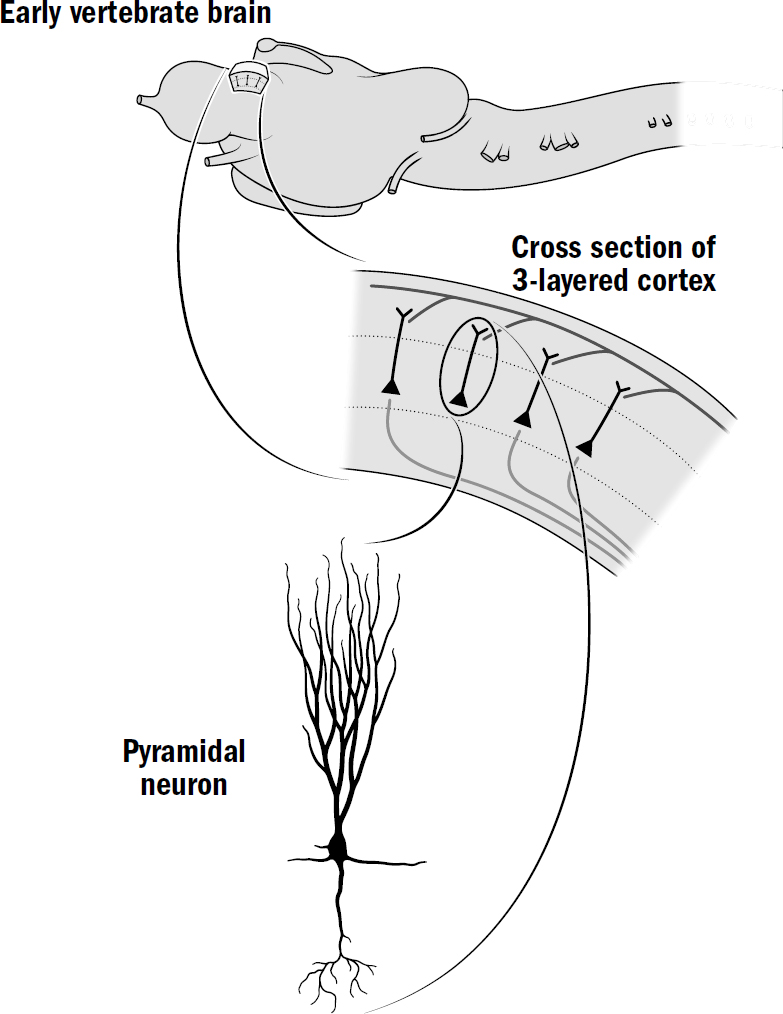
Figure 7.5: The cortex of early vertebrates
Original art by Rebecca Gelernter
The Cortex
In the first cortex evolved a new morphology of neuron, the pyramidal neuron, named after their pyramid-like shape. These pyramidal neurons have hundreds of dendrites and receive inputs across thousands of synapses. These were the first neurons designed for the purpose of recognizing patterns.
Olfactory neurons send their signals to the pyramidal neurons of the cortex. This network of olfactory input to the cortex has two interesting properties. First, there is a large dimensionality expansion—a small number of olfactory neurons connect to a much larger number of cortical neurons. Second, they connected sparsely; a given olfactory cell will connect to only a subset of these cortical cells. These two seemingly innocuous features of wiring may solve the discrimination problem.
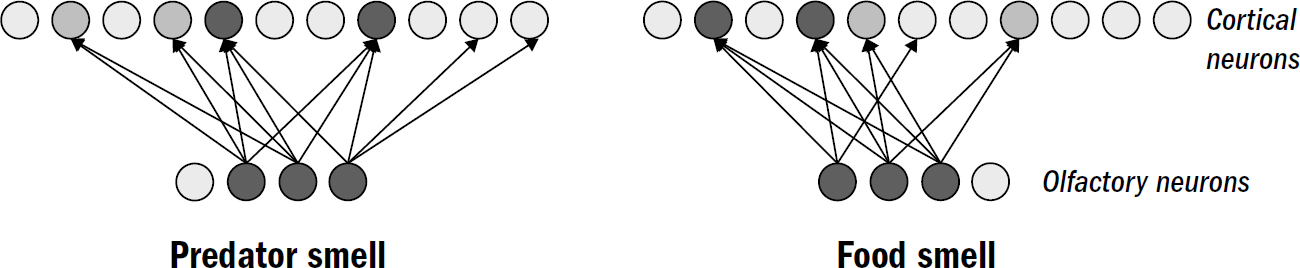
Figure 7.6: Expansion and sparsity (also called expansion recoding) can solve the discrimination problem
Original art by Rebecca Gelernter
Neuroscientists have also found hints of how the cortex might solve the problem of generalization. Pyramidal cells of the cortex send their axons back onto themselves, synapsing on hundreds to thousands of other nearby pyramidal cells. This means that when a smell pattern activates a pattern of pyramidal neurons, this ensemble of cells gets automatically wired together through Hebbian plasticity.* The next time a pattern shows up, even if it is incomplete, the full pattern can be reactivated in the cortex. This trick is called auto-association; neurons in the cortex automatically learn associations with themselves. This offers a solution to the generalization problem—the cortex can recognize a pattern that is similar but not the same.
Auto-association reveals an important way in which vertebrate memory differs from computer memory. Auto-association suggests that vertebrate brains use content-addressable memory—memories are recalled by providing subsets of the original experience, which reactivate the original pattern. If I tell you the beginning of a story you’ve heard before, you can recall the rest; if I show you half a picture of your car, you can draw the rest. However, computers use register-addressable memory—memories that can be recalled only if you have the unique memory address for them. If you lose the address, you lose the memory.
Auto-associative memory does not have this challenge of losing memory addresses, but it does struggle with a different form of forgetfulness. Register-addressable memory enables computers to segregate where information is stored, ensuring that new information does not overwrite old information. In contrast, auto-associative information is stored in a shared population of neurons, which exposes it to the risk of accidentally overwriting old memories. Indeed, as we will see, this is an essential challenge with pattern recognition using networks of neurons.
Catastrophic Forgetting (or The Continual Learning Problem, Part 2)
In 1989, Neal Cohen and Michael McCloskey were trying to teach artificial neural
Cohen and McCloskey converted numbers into patterns of neurons, then trained a neural network to do addition by transforming two input numbers (e.g., 1 and 3) into the correct output number (in this case, 4). They first taught the network to add ones (1+2, 1+3, 1+4, and so on) until it got good at it. Then they taught the same network to add twos (2+1, 2+2, 2+3, and so on) until it got good at this as well.
But then they noticed a problem. After they taught the network to add twos, it forgot how to add ones. When they propagated errors back through the network and updated the weights to teach it to add twos, the network had simply overridden the memories of how to add ones. It successfully learned the new task at the expense of the previous task.
Cohen and McCloskey referred to this property of artificial neural networks as the problem of catastrophic forgetting. This was not an esoteric finding but a ubiquitous and devastating limitation of neural networks: when you train a neural network to recognize a new pattern or perform a new task, you risk interfering with the network’s previously learned patterns.
How do modern AI systems overcome this problem? Well, they don’t yet. Programmers merely avoid the problem by freezing their AI systems after they are trained. We don’t let AI systems learn things sequentially; they learn things all at once and then stop learning.
The artificial neural networks that recognize faces, drive cars, or detect cancer in radiology images do not learn continually from new experiences. As of this book going to print, even ChatGPT, the famous chatbot released by OpenAI, does not continually learn from the millions of people who speak to it. It too stopped learning the moment it was released into the world. These systems are not allowed to learn new things because of the risk that they will forget old things (or learn the wrong things). So modern AI systems are frozen in time, their parameters locked down; they are allowed to be updated only when retrained from scratch with humans meticulously monitoring their performance on all the relevant tasks.
The humanlike artificial intelligences we strive to create are, of course, not like this. Rosey from The Jetsons learned as you spoke to her—you could show her how to play a game and she could then play it without forgetting how to play other games.
While we are only just beginning to explore how to make continual learning work, animal brains have been doing so for a long time.
As soon as pattern recognition evolved, so too did a solution to the problem of catastrophic forgetting. Indeed, even fish avoid catastrophic forgetting fantastically well. Train a fish to escape from a net through a small escape hatch, leave the fish alone for an entire year, and then test it again. During this long stretch of time, its brain will have received a constant stream of patterns, learning continually to recognize new smells, sights, and sounds. And yet, when you place the fish back in the same net an entire year later, it will remember how to get out with almost the same speed and accuracy as it did
There are several theories about how vertebrate brains do this. One theory is that the cortex’s ability to perform pattern separation shields it from the problem of catastrophic forgetting; by separating incoming patterns in the cortex, patterns are inherently unlikely to interfere with each other.
We do not yet understand exactly how simple vertebrate brains, like those of fish, reptiles, and amphibians, are capable of overcoming the challenges of catastrophic forgetting. But the next time you spot a fish, you will be in the presence of the answer, hidden in its small cartilaginous head.
The Invariance Problem
Look at the two objects below.
As you view each object, a specific pattern of neurons in the backs of your eyes light up. The minuscule half-millimeter-thick membrane in the back of the eye—the retina—contains over one hundred million neurons of five different types. Each region of the retina receives input from a different location of the visual field, and each type of neuron is sensitive to different colors and contrasts. As you view each object, a unique pattern of neurons activates a symphony of spikes. Like the olfactory neurons that make up a smell pattern, the neurons in the retina make up a visual pattern; your ability to see exists only in your ability to recognize these visual patterns.
Figure 7.7
Free 3D objects found on SketchFab.com
The activated neurons in the retina send their signals to the thalamus, which then sends these signals to the part of the cortex that processes visual input (the visual cortex). The visual cortex decodes and memorizes the visual pattern the same way the olfactory cortex decodes and memorizes smell patterns. This is, however, where the similarity between sight and smell ends.
Look at the objects below. Can you identify which shapes are the same as the ones in the first picture?
Figure 7.8
Free 3D objects found on SketchFab.com
The pattern of olfactory neurons activated by the smell of an egg is the same no matter the rotation, distance, or location of the egg. The same molecules diffuse through the air and activate the same olfactory neurons. But this is not the case for other senses such as vision.
The same visual object can activate different patterns depending on its rotation, distance, or location in your visual field. This creates what is called the invariance problem: how to recognize a pattern as the same despite large variances in its inputs.
Nothing we have reviewed about auto-association in the cortex provides a satisfactory explanation for how the brain so effortlessly did this. The auto-associative networks we described cannot identify an object you have never seen before from completely different angles. An auto-associative network would treat these as different objects because the input neurons are completely different.
This is not only a problem with vision. When you recognize the same set of words spoken by the high-pitched voice of a child and the low-pitched voice of an adult, you are solving the invariance problem. The neurons activated in your inner ear are different because the pitch of the sound is completely different, and yet you can still tell they are the same words. Your brain is somehow recognizing a common pattern despite huge variances in the sensory input.
In 1958, decades before Cohen and McCloskey discovered the problem of catastrophic forgetting, a different team of neuroscientists, also at Johns Hopkins, were exploring a different aspect of pattern recognition.
David Hubel and Torsten Wiesel anesthetized cats, put electrodes into their cortices, and recorded the activity of neurons as they
In mammal brains (cats, rats, monkeys, humans, et cetera), the part of the cortex that first receives input from the eye is called V1 (the first visual area). Hubel and Wiesel discovered that individual neurons in V1 were surprisingly selective with what they respond to. Some neurons were activated only by vertical lines at a specific location in a cat’s visual field. Other neurons were activated only by horizonal lines at some other location, and still others by 45-degree lines at a different location. The entire surface area of V1 makes up a map of the cat’s full field of view, with individual neurons selective for lines of specific orientations at each location.
Figure from Manassi et al., 2013. Used with permission.
V1 decomposes the complex patterns of visual input into simpler features, like lines and edges. From here, the visual system creates a hierarchy: V1 sends its output to a nearby region of cortex called V2, which then sends information to an area called V4, which then sends information to an area called IT.
Neurons at progressively higher levels of this cortical hierarchy become sensitive to progressively more sophisticated features of visual stimuli—neurons in V1 are primarily activated by basic edges and lines, neurons in V2 and V4 are sensitive to more complex shapes and objects, and neurons in IT are sensitive to complex whole objects such as specific faces. A neuron in V1 responds only to input in a specific region of one’s visual field; in contrast, a neuron in IT can detect objects across any region of the eye. While V1 decomposes pictures into simple features, as visual information flows up the hierarchy, it is pieced back together into whole objects.
In the late 1970s, well over twenty years after Hubel and Wiesel’s initial work, a computer scientist by the name of Kunihiko Fukushima was trying to get computers to recognize objects in pictures. Despite his best attempts, he couldn’t get standard neural networks, like those depicted earlier in the chapter, to successfully do it; even small changes in the location, rotation, or size of an object activated entirely different sets of neurons, which blinded networks to generalizing different patterns to the same object—a square over here would be incorrectly perceived as different from the same square over there. He had stumbled on the invariance problem. And he knew that somehow, brains solved it.
Fukushima had spent the prior four years working in a research group that included several neurophysiologists, and so he was familiar with the work of Hubel and Wiesel. Hubel and Wiesel had discovered two things. First, visual processing in mammals was hierarchical, with lower levels having smaller receptive fields and recognizing simpler features, and higher levels having larger receptive fields and recognizing more complex objects. Second, at a given level of the hierarchy, neurons were all sensitive to similar features, just in different places. For example, one area of V1 would look for lines at one location, and another area would look for lines for another location, but they were all looking for lines.
After these feature maps identified certain features, their output was compressed and passed to another set of feature maps that could combine them into higher-level features across a wider area of the picture, merging lines and edges into more complex objects. All this was designed to be analogous to the visual processing of the mammalian cortex. And, amazingly, it worked.
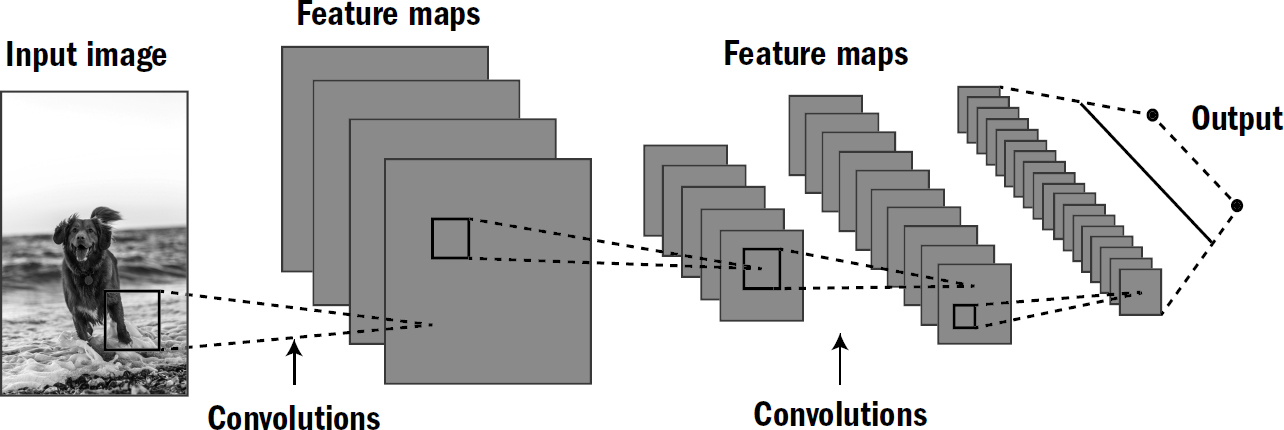
Figure 7.10: A convolutional neural network
Figure designed by Max Bennett. The dog photo is from Oscar Sutton (purchased on Unsplash).
Most modern AI systems that use computer vision, from your self-driving car to the algorithms that detect tumors in radiology images use Fukushima’s convolutional neural networks. AI was blind, but now can see, a gift that can be traced all the way back to probing cat neurons over fifty years ago.
The brilliance of Fukushima’s convolutional neural network is that it imposes a clever “inductive bias.” An inductive bias is an assumption made by an AI system by virtue of how it is designed. Convolutional neural networks are designed with the assumption of translational invariance, that a given feature in one location should be treated the same as that same feature but in a different location. This is an impregnable fact of our visual world: the same thing can exist in different places without the thing being different. And so, instead of trying to get an arbitrary web of neurons to learn this fact about the visual world, which would require too much time and data, Fukushima simply encoded this rule directly into the architecture of the network.
Despite being inspired by the brain, convolutional neural networks (CNNs) are, in fact, a poor approximation of how brains recognize visual patterns. First, visual processing isn’t as hierarchical as originally thought; input frequently skips levels and branches out to multiple levels simultaneously. Second, CNNs impose the constraint of translation, but they don’t inherently understand rotations of 3D objects, and thus don’t do a great job recognizing objects when rotated.* Third, modern CNNs are still founded on supervision and backpropagation—with its magical simultaneous updating of many connections—while the cortex seems to recognize objects without supervision and without backpropagation.
And fourth, and perhaps most important, CNNs were inspired by the mammal visual cortex, which is much more complex than the simpler visual cortex of fish; and yet the fish brain—lacking any obvious hierarchy or the other bells and whistles of the mammalian cortex—is still eminately capable of solving the invariance problem.
In 2022, the comparative psychologist Caroline DeLong at Rochester Institute of Technology trained goldfish to
Perhaps the best lesson from CNNs is not the success of the specific assumptions they attempt to emulate—such as translational invariance—but the success of assumptions themselves. Indeed, while CNNs may not capture exactly how the brain works, they reveal the power of a good inductive bias. In pattern recognition, it is good assumptions that make learning fast and efficient. The vertebrate cortex surely has such an inductive bias, we just don’t know what it is.
In some ways, the tiny fish brain surpasses some of our best computer-vision systems. CNNs require incredible amounts of data to understand changes in rotations and 3D objects, but a fish seems to recognize new angles of a 3D object in one shot.
In the predatory arms race of the Cambrian, evolution shifted from arming animals with new sensory neurons for detecting specific things to arming animals with general mechanisms for recognizing anything.
With this new ability of pattern recognition, vertebrate sensory organs exploded with complexity, quickly flowering into their modern form. Noses evolved to detect chemicals; inner ears evolved to detect frequencies of sound; eyes evolved to detect sights. The coevolution of the familiar sensory organs and the familiar brain of vertebrates is not a coincidence—they each facilitated the other’s growth and complexity. Each incremental improvement to the brain’s pattern recognition expanded the benefits to be gained by having more detailed sensory organs; and each incremental improvement in the detail of sensory organs expanded the benefits to be gained by more sophisticated pattern recognition.
In the brain, the result was the vertebrate cortex, which somehow recognizes patterns without supervision, somehow accurately discriminates overlapping patterns and generalizes patterns to new experiences, somehow continually learns patterns without suffering from catastrophic forgetting, and somehow recognizes patterns despite large variances in its input.
The elaboration of pattern recognition and sensory organs, in turn, also found themselves in a feedback loop with reinforcement learning itself. It is also not a coincidence that pattern recognition and reinforcement learning evolved simultaneously in evolution. The greater the brain’s ability to learn arbitrary actions in response to things in the world, the greater the benefit to be gained from recognizing more things in the world. The more unique objects and places a brain can recognize, the more unique actions it can learn to take. And so the cortex, basal ganglia, and sensory organs evolved together, all emerging from the same machinations of reinforcement learning.
8
Why Life Got Curious
IN THE AFTERMATH of the success of TD-Gammon, researchers began applying Sutton’s temporal difference learning to all kinds of different games. And one by one, games that had previously been “unsolvable” were successfully beaten by these algorithms; TD learning algorithms eventually surpassed human-level performance in video games like Pinball, Star Gunner, Robotank, Road Runner, Pong, and Space Invaders. And yet there was one Atari game that was perplexingly out of reach:
In Montezuma’s Revenge, you start in a room filled with obstacles. In each direction is another room, each with its own obstacles. There is no sign or clue as to which direction is the right way to go. The first reward is earned when you find your way to a hidden door in a faraway hidden room. This makes the game particularly hard for reinforcement learning systems: the first reward occurs so late in the game that there is no early nudging of what behavior should be reinforced or punished. And yet somehow, of course, humans beat this game.
It wasn’t until 2018 when an algorithm was developed that finally completed level one of Montezuma’s Revenge. This new algorithm, developed by Google’s DeepMind, accomplished this feat by adding something familiar that was missing from Sutton’s original TD learning algorithm: curiosity.
Sutton had always known that a problem with any reinforcement learning system is something called the exploitation-exploration dilemma. For trial-and-error learning to work, agents need to, well, have lots of trials from which to learn. This means that reinforcement learning can’t work by just exploiting behaviors they predict lead to rewards; it must also explore new behaviors.
In other words, reinforcement learning requires two opponent processes—one for behaviors that were previously reinforced (exploitation) and the other for behaviors that are new (exploration). These choices are, by definition, opposing each other. Exploitation will always drive behavior toward known rewards, and exploration will always drive toward what is unknown.
In early TD learning algorithms, this trade-off was implemented in a crude way: these AI systems spontaneously—say, 5 percent of the time—did something totally random. This worked okay if you were playing a constrained game with only so many next moves, but it worked terribly in a game like Montezuma’s Revenge, where there were practically an infinite number of directions and places you could go.
There is an alternative approach to tackling the exploitation-exploration dilemma, one that is both beautifully simple and refreshingly familiar. The approach is to make AI systems explicitly curious, to reward them for exploring new places and doing new things, to make surprise itself reinforcing. The greater the novelty, the larger the compulsion to explore it. When AI systems playing Montezuma’s Revenge were given this intrinsic motivation to explore new things, they behaved very differently—indeed, more like a human player. They became motivated to explore areas, go to new rooms, and expand throughout the map. But instead of exploring through random actions, they explored deliberately; they specifically wanted to go to new places and to do new things.
Even though there are no explicit rewards until you get past all the rooms in level one, these AI systems didn’t need any external rewards to explore. They were motivated on their own. Simply finding their way to a new room was valuable in and of itself. Armed with curiosity, suddenly these models started making progress, and they eventually beat level one.
The emergence and mechanisms of curiosity help explain gambling, which is an irrational oddity of vertebrate behavior. Gamblers violate Thorndike’s law of effect—they continue to gamble their money away despite the fact that the expected reward is negative.
One explanation for this is that vertebrates get an extra boost of reinforcement when something is surprising. To make animals curious, we evolved to find surprising and novel things reinforcing, which drives us to pursue and explore them. This means that even if the reward of an activity is negative, if it is novel, we might pursue it anyway.
Games of gambling are carefully designed to exploit this. In games of gambling, you don’t have a 0 percent chance of winning (which would lead you not to play); you have a 48 percent chance of winning, high enough to make it possible, uncertain enough to make it surprising when you win (giving you a dopamine boost), and low enough so that the casino will, in the long run, suck you dry.
Our Facebook and Instagram feeds exploit this as well. With each scroll, there is a new post, and randomly, after some number of scrolls, something interesting shows up. Even though you might not want to use Instagram, the same way gamblers don’t want to gamble or drug addicts don’t want to use anymore, the behavior is subconsciously reinforced, making it harder and harder to stop.
Gambling and social feeds work by hacking into our five-hundred-million-year-old preference for surprise, producing a maladaptive edge case that evolution has not had time to account for.
Curiosity and reinforcement learning coevolved because curiosity is a requirement for reinforcement learning to work. With the newfound ability to recognize patterns, remember places, and flexibly change behavior based on past rewards and punishments, the first vertebrates were presented with a new opportunity: for the first time, learning became, in and of itself, an extremely valuable activity. The more patterns a vertebrate recognized and the more places she remembered, the better she would survive. And the more new things she tried, the more likely she was to learn the correct contingencies between her actions and their corresponding outcomes. And so it was 500 million years ago in the tiny brain of our fish-like ancestors when curiosity first emerged.
9
The First Model of the World
HAVE YOU EVER tried to navigate through your home in the dark? I’m guessing not on purpose, but perhaps during a power outage or a midnight stroll to the bathroom. If you have ever tried this, you probably had the (not very surprising) realization that it is hard to do. As you step out of your bedroom and walk toward the end of the hall, you are prone to mis-predicting the length of the hallway or the exact location of the bathroom door. You might stub a toe.
But you would also notice that, despite your blindness, you have a reasonable hunch about where the end of the hallway is, some intuition about where you are in the labyrinth of your home. You might be off by a step or two, but your intuition nonetheless proves an effective guide. What is remarkable about this is not that it is hard, but that it is achievable at all.
The reason you can do this is that your brain has built a spatial map of your home. Your brain has an internal model of your home, and thus, as you move, your brain can update your position in this map on its own. This trick, the ability to construct an internal model of the external world, was inherited from the brains of first vertebrates.
The Maps of Fish
This same find-your-way-to-the-bathroom-in-the-dark test can be done in fish. Well, not the bathroom part, but the general test of remembering a location without a visual guide. Put a fish in an empty tank with a grid of twenty-five identical containers throughout the tank. Hide food in one of the containers. The fish will explore the tank, randomly inspecting each container until it stumbles on the food. Now take the fish out of the tank, put food back in the same container, and put the fish back into the tank. Do this a few times, and the fish will learn to quickly dart
Fish are not learning some fixed rule of Always turn left when I see this object—they navigate to the correct location no matter where in the tank they are initially placed. And they are not learning some fixed rule of Swim toward this image or smell of food; fish will go back to the correct container even if you don’t put any food back in the container. In other words, even if every container is exactly identical because none of them have any food at all, fish still correctly identify which container is currently placed at the exact location that previously contained food.
The only clue as to which container previously held the food was the walls of the tank itself, which had markings to designate specific sides. Thus, fish somehow identified the correct container based solely on the container’s location relative to the landmarks on the side of the tank. The only way fish could have accomplished this is by building a spatial map—an internal model of the world—in their minds.
Your Inner Compass
Here’s another test you can do on yourself: Sit in one of those swivel chairs, close your eyes, ask someone to turn the chair, and then guess what direction of the room you are facing before opening your eyes. You will be amazingly accurate. How did your brain do this?
Deep in your inner ear are semicircular canals, small tubes filled with fluid. These canals are lined with sensory neurons that float in this fluid and activate whenever they detect movement. The semicircular canals are organized in three loops, one for facing forward, one for facing sideways, and one for facing upward. The fluid in each of these canals moves only when you move in that specific dimension. Thus, the ensemble of activated sensory cells signal the direction of head movement. This creates a unique sense—the vestibular sense. This is why you get dizzy if you are spun in a chair continuously—eventually, this overactivates these sensory cells and when you stop turning, they are still active, incorrectly signaling rotation even when you aren’t rotating.
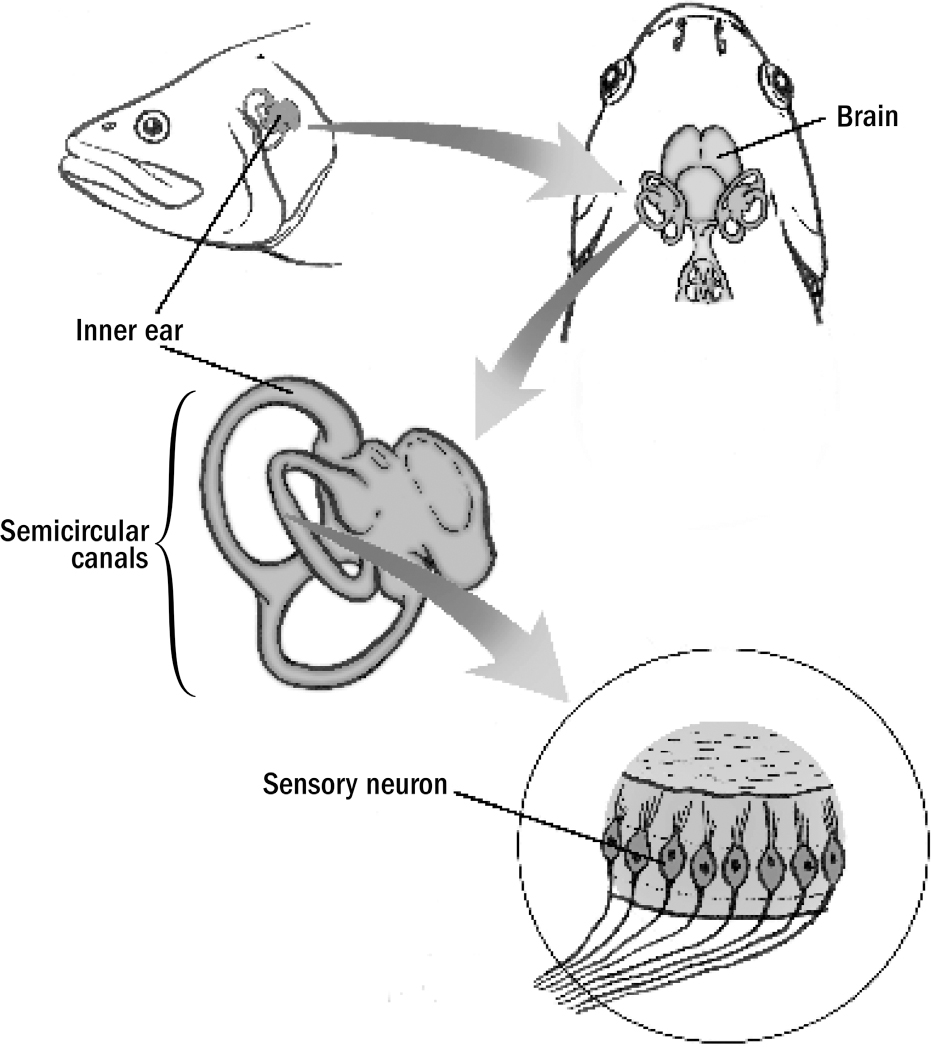
Image by Carlyn Iverson / Science Source. Used with permission.
The evolutionary origin of semicircular canals was in early vertebrates, emerging at the same time as reinforcement learning and the ability to construct spatial maps. Modern fish have the same structure in their inner ears, and it enables them to identify when and by how much they are moving.
The vestibular sense is a necessary feature of building a spatial map. An animal needs to be able to tell the difference between something swimming toward it and it swimming toward something. In each case, the visual cues are the same (both show an object coming closer), but each means very different things in terms of movement through space. The vestibular system helps the fish tell the difference: If it starts swimming toward an object, the vestibular system will detect this acceleration. In contrast, if an object starts moving toward it, no such activation will occur.
But if the hindbrain of fish constructs a compass of an animal’s own direction, where is the model of external space constructed? Where does the vertebrate brain store information about the locations of things relative to other things?
The Medial Cortex (aka the Hippocampus)
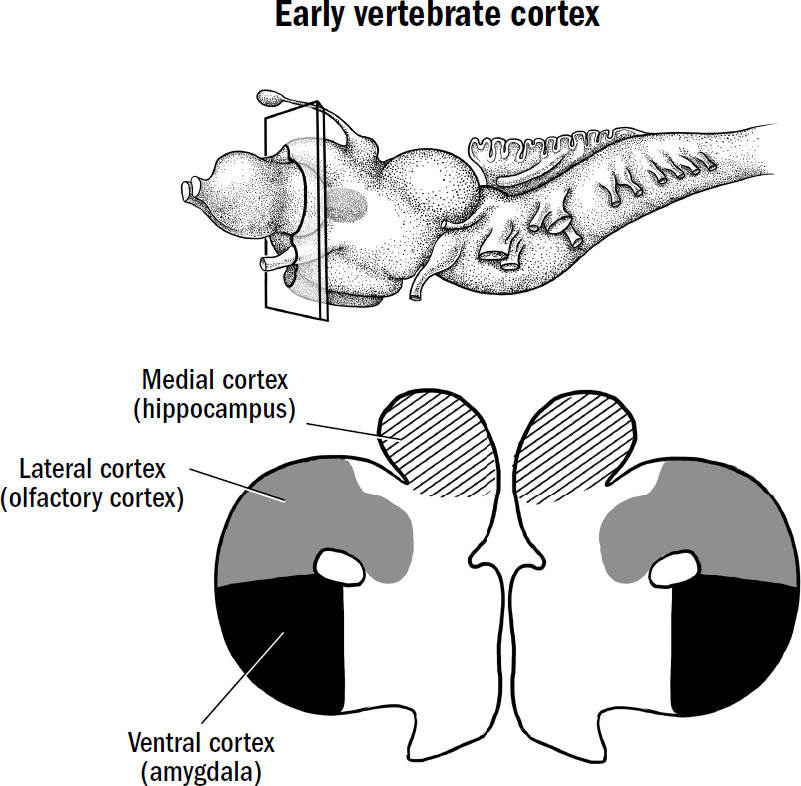
Figure 9.2: The cortex of early vertebrates
Original art by Mesa Schumacher
Clearly the three-layered cortex of early vertebrates performed computations far beyond simple auto-association. Not only is it also seemingly capable of recognizing objects despite large changes in rotation and scale (solving the invariance problem), but it is also seemingly capable of constructing an internal model of space. To speculate: Perhaps the ability of the cortex to recognize objects despite changes in rotation and its ability to model space are related. Perhaps the cortex is tuned to model 3D things—whether those things are objects or spatial maps.
The evolution of spatial maps in the minds of early vertebrates marked numerous firsts. It was the first time in the billion-year history of life that an organism could recognize where it was. It is not hard to envision the advantage this would have offered. While most invertebrates steered around and executed reflexive motor responses, early vertebrates could remember the places where arthropods tended to hide, how to get back to safety, and the locations of nooks and crannies filled with food.
It was also the first time a brain differentiated the self from the world. To track one’s location in a map of space, an animal needs to be able to tell the difference between “something swimming toward me” and “me swimming toward something.”
And most important, it was the first time that a brain constructed an internal model—a representation of the external world. The initial use of this model was, in all likelihood, pedestrian: it enabled brains to recognize arbitrary locations in space and to compute the correct direction to a given target location from any starting location. But the construction of this internal model laid the foundation for the next breakthrough in brain evolution. What began as a trick for remembering locations would go on to become much more.
Summary of Breakthrough #2: Reinforcing
Our ancestors from around five hundred million years ago transitioned from simple wormlike bilaterians to fishlike vertebrates. Many new brain structures and abilities emerged in these early vertebrate brains, most of which can be understood as enabling and emerging from breakthrough #2: reinforcement learning. These include
- Dopamine became a temporal difference learning signal, which helped solve the temporal credit assignment problem and enabled animals to learn through trial and error.
- The basal ganglia emerged as an actor-critic system, enabling animals to generate this dopamine signal by predicting future rewards and to use this dopamine signal to reinforce and punish behaviors.
- Curiosity emerged as a necessary part of making reinforcement learning work (solving the exploration-exploitation dilemma).
- The cortex emerged as an auto-associative network, making pattern recognition possible.
- The perception of precise timing emerged, enabling animals to learn, through trial and error, not only what to do but when to do it.
- The perception of three-dimensional space emerged (in the hippocampus and other structures), enabling animals to recognize where they were and remember the location of things relative to other things.
Reinforcement learning in early vertebrates was possible only because the mechanisms of valence and associative learning had already evolved in early bilaterians. Reinforcement learning is bootstrapped on simpler valence signals of good and bad. Conceptually, the vertebrate brain is built on top of the more ancient steering system of bilaterians. Without steering, there is no starting point for trial and error, no foundation on which to measure what to reinforce or un-reinforce.
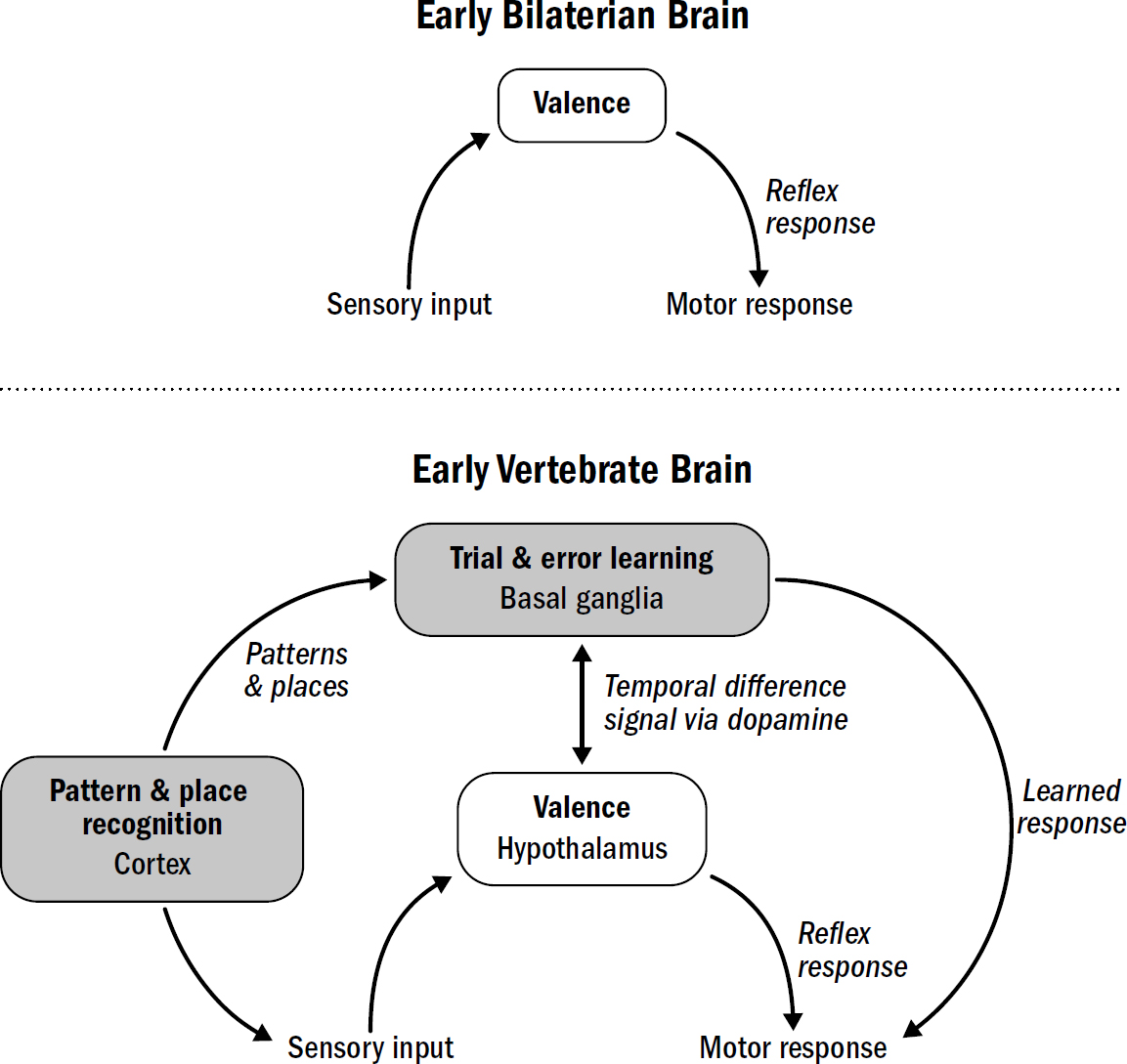
Figure 9.3
Original art by Rebecca Gelernter
Steering bilaterians made it possible for later vertebrates to learn through trial and error. And trial and error in vertebrates, in turn, made it possible for the even more perplexing and monumental breakthrough that would follow. It was early mammals who first figured out how to engage in a different flavor of trial and error: learning not by doing but by imagining.
Breakthrough #3
Simulating and the First Mammals

Your brain 200 million years ago
Original art by Rebecca Gelernter
10
The Neural Dark Ages
FROM 420 TO 375 million years ago, oceans became filled with progressively more diverse predatory fish of many shapes and sizes. What would have resembled the sharks and stingrays of today were common sightings. Twenty-foot-long placoderms, fish with armored head plates and thick bone-crushing teeth, found themselves at the top of this food chain.
Arthropods and other invertebrates were relegated to various niches. Some got smaller. Some evolved thicker shells. Some even took a cue from early vertebrates and survived by getting smarter—it was during this period that the cephalopods emerged, the ancestors of today’s squids and octopuses. Under severe pressure to survive their mass hunting by fish, cephalopods became impressively intelligent down an independent lineage with brains that work very differently than our own.
The most radical invertebrate survival strategy was to escape the sea all together. The arthropods, driven from their homeland by relentless predation, were the first animals to walk out of the oceans and populate the land. They found respite among the small leafless land-faring plants that had sparsely sprouted along the seashores.
The period between 420 to 375 million years ago is called the Devonian period, and it was here when land plants first evolved leaves for better absorption of sunlight and seeds for spreading, both of which enabled plants to propagate
While life for arthropods was horrifying in the sea, it was heavenly on land. Arthropods developed new tricks to meet the needs of life on land, diversifying into what resembled today’s spiders and insects. Unfortunately, as we have seen with today’s problem of climate change, Earth’s biosphere is unforgiving to those who proliferate rapidly and unsustainably. What began as a small oasis for arthropod refugees eventually became an overextended orgy of plant life, triggering a global extinction event that would eradicate close to half of all life.
A Story of Two Great Deaths
History repeats itself.
One and a half billion years ago, the explosion of cyanobacteria suffocated the Earth with carbon dioxide and polluted it with oxygen. Over a billion years later, the explosion of plants on land seems to have committed a similar crime.
Extinction events create opportunities for small niches to transform into dominant strategies. Before the Late Devonian Extinction, our ancestors had found such a niche. Most fish stayed far away from the shore to avoid deadly beaching, a situation where a fish becomes stuck on land as tides recede. Although the risk of beaching made it dangerous to pursue, there was a big nutritional prize to be found close to the shore: the warm earthy puddles were full of small insects and vegetation.
Our ancestors were the first fish to evolve the ability to survive out of water. They developed a pair of lungs that augmented their gills, enabling them to extract oxygen from both water and air. And so our ancestors would use their fins both for swimming in water and for wading themselves short distances on land, traveling from puddle to puddle in search of insects.
When the Late Devonian Extinction Event began to freeze over the oceans, our air-breathing and land-walking ancestors were one of the few warm-water fish to survive. As the food supply in warm waters began to die, our ancestors spent more of their time living in the inland puddles. They lost their gills (and thus their ability to breath underwater), and their webbed fins gave way to fingered hands and feet. They became the first tetrapods (tetra for “four” and pods for “feet”), most closely resembling a modern amphibian such as a salamander.
One evolutionary lineage of tetrapods, who were lucky enough to live in parts of the Earth that still supported these warmer puddles, would maintain this lifestyle for hundreds of millions of years—they would become the amphibians of today. Another lineage abandoned the dying shores and wandered farther inland in search of food. This was the lineage of amniotes—the creatures that developed the ability to lay leathery eggs that could survive out of the water.
The first amniotes probably best resembled a lizard of today. Amniotes found an inland ecosystem abundant with food—insects and plants were everywhere for the feasting. Eventually, the Devonian ice age faded and amniotes spread and diversified to all corners of the Earth. The Carboniferous and Permian eras, which collectively lasted from 350 million years ago to 250 million years ago, saw an explosion of amniotes on land.
Living on land presented unique challenges to the amniotes that their fish cousins never faced. One such challenge was temperature fluctuations. Cycles of the day and season create only muted temperature changes deep in the oceans. In contrast, temperatures can fluctuate dramatically on the surface. Amniotes, like fish, were cold-blooded—their only strategy for regulating their body temperature was to physically relocate to warmer places.
One amniote lineage were the reptiles, who would eventually diversify into dinosaurs, lizards, snakes, and turtles. Most of these reptiles dealt with daily temperature fluctuations by becoming immobile at night. Temperatures were too low for their muscles and metabolisms to function properly, so they simply shut down. The fact that reptiles were shut down for a third of their lives presented an opportunity—creatures that could hunt in the night would reap an incredible feast of motionless lizards.
In the Permian era, when the land was full of edible reptiles and arthropods, this gamble paid off. During the period from 300 to 250 million years ago, therapsids became the most successful land animals. They grew to the size of a modern tiger and began to grow hair to further maintain their heat. These therapsids would have looked like large hairy lizards.
Perhaps you can already see a trend emerging from the evolutionary history of life on Earth: all reigns come to an end. The therapsid reign on Earth was no different: the Permian-Triassic mass extinction event, which occurred around 250 million years ago, was the deadliest of all extinction events in Earth’s history. It was the second great death in this era. This extinction event was the most severe and perhaps the most enigmatic. Within five to ten million years, 96 percent of all marine life died, and 70 percent of all land life died. There is still controversy over what caused this—theories include asteroids, volcanic explosions, and methane-producing microbes. Some suggest that it was no single reason but rather a perfect storm of multiple unlucky occurrences. Regardless of the cause, we know the effects.
The large therapsids went almost entirely extinct. The gamble of warm-bloodedness that originally facilitated their rise was also the cause of their downfall. During a period of reduced access to food, the therapsids, with their need for huge amounts of calories, died first. The reptiles and their comparatively scant diets were much better suited to weather this storm.
Figure 10.1: The first therapsid
Original art by Rebecca Gelernter
For about five million years, life survived only in tiny pockets of the world. The only therapsids that survived were the small plant-eating ones, such as the burrowing cynodonts. The cynodonts originally evolved into the niche of burrowing underground to hide from the larger and more predatory therapsids that dominated the world. As food supply went away and all those bigger animals died off, these small cynodonts were among the few surviving therapsids to emerge on the other side of the Permian-Triassic extinction.
Although the therapsid lineage was just barely preserved by the small cynodont, the world they found themselves in was different. On the other side of this extinction event, with 70 percent of land life extinguished, reptiles emerged numerous, diverse, and big. The eradication of the large therapsids handed the animal kingdom to their scaly reptilian cousins. From the end of this extinction event and for the next one hundred fifty million years, reptiles would rule.
Small lizards of the Permian evolved into twenty-foot-long predatory archosaurs with massive teeth and claws, resembling a smaller Tyrannosaurus. It was also during this period that vertebrates took to the skies—the pterosaur, a flying archosaur, was the first to grow wings and hunt from above.
In order to survive this ravenous era of predatory dinosaurs, pterosaurs, and other massive reptilian beasts, cynodonts got smaller and smaller until they were no more than four inches long. Equipped with warm-bloodedness and miniaturization, they survived by hiding in burrows during the day and emerging during the cold night when archosaurs were relatively blind and immobile. They made their homes in dug-out burrowed mazes or in the thick bark of trees. They hunted by quietly wandering the twilight forest floors and tree branches in search of insects. They became the first mammals.
Figure 10.2: The evolutionary tree from the first vertebrates to the first mammals. MYA = million years ago.
Original art by Rebecca Gelernter
At some point in this hundred-million-year reign of dinosaurs, as these small mammals survived tucked away in nooks and crannies of the world, they added one more survival trick to their repertoire. They evolved a new cognitive ability, the biggest neural innovation since the Cambrian fish.
Surviving by Simulating
This early four-inch-long mammal, likely resembling a mouse or squirrel of today, was not stronger than dinosaurs or birds and surely unable to fight its way out of a predatory assault. It was probably also slower, or at least no faster, than an archosaur or a pterosaur swooping down from the sky. But the burrowing and arboreal lifestyle did indeed give early mammals a singular advantage: they got to make the first move. From an underground burrow or from behind a tree branch, they got to look around, spot a faraway bird and a tasty insect, and decide whether to make a run for it. This gift of the first move was left unexploited for hundreds of millions of years. But eventually a neural innovation emerged to exploit it: a region of the cortex transformed, through a currently unknown series of events, into a new region called the neocortex (neo for “new”).
The neocortex gave this small mouse a superpower—the ability to simulate actions before they occurred. It could look out at a web of branches leading from its hole to a tasty insect. It could see the faraway eyes of a nearby predatory bird. The mouse could simulate going down different paths, simulate the bird chasing it and the insects hopping away, then pick the best path—the one that, in its simulation, it found itself both alive and well fed. If the reinforcement-learning early vertebrates got the power of learning by doing, then early mammals got the even more impressive power of learning before doing—of learning by imagining.
Many creatures had previously found themselves in positions of having the first move—crabs hide under sand and small fish weave between the leaves of coral plants. So then why was it only with mammals that simulating emerged?
It has been speculated that there were two requirements for simulating to evolve. First, you need far-ranging vision—you need to be able to see a lot of your surroundings in order for simulating paths to be fruitful. On land, even at night, you can see up to one hundred times farther
Inside the Brain of the First Mammals
Throughout this several-hundred-million-year-long story, from the emergence of fish onto land to the rise of dinosaurs, there was an expansive diversification of animal shapes, sizes, and organs. And yet, there was one thing that was surprisingly unchanged: brains.
It was only in early mammals that a spark of innovation emerged from the eternity of neural stagnation. The fish cortex split into four separate structures in early mammals, three of which were effectively the same as the subregions that had come before, only one of which, the neocortex, could truly be considered new. The ventral cortex of early vertebrates became the associative amygdala in mammals, containing similar circuitry and serving largely the same purpose: learning to recognize patterns across various modalities, especially those that were predictive of valence outcomes (e.g., predicting that sound A leads to good things and sound B leads to bad things). The smell-pattern detectors in the lateral cortex of early vertebrates became the olfactory cortex in mammals, working the same way—detecting smell patterns through auto-associative networks. The medial cortex of early vertebrates, where spatial maps were learned, became the hippocampus of mammals, performing a similar function using similar circuitry. But a fourth region of the cortex underwent a more meaningful change—it transformed into the neocortex, which contained completely different circuitry.
Other than the emergence of the neocortex, the brain of early mammals was largely the same as that of early vertebrates. The basal ganglia integrated input about the world from the olfactory cortex, hippocampus, amygdala, and now also the neocortex to learn to take actions that maximized dopamine release. The hypothalamus still triggered direct valence responses and modulated other structures through neuromodulators such as dopamine. Midbrain and hindbrain structures still implemented reflexive movement patterns, albeit now specialized for walking as opposed to swimming.
Original art by Rebecca Gelernter
The neocortex of this early mammal was small and took up only a small fraction of the brain. Most volume was given to the olfactory cortex (early mammals, like many modern mammals, had an incredible sense of smell). But despite the small size of the neocortex in early mammals, it was still the kernel from which human intelligence would arise. In the human brain, the neocortex takes up 70 percent of brain volume. In the breakthroughs that followed, this originally small structure would progressively expand from a clever trick to the epicenter of intelligence.
11
Generative Models and the Neocortical Mystery
WHEN YOU LOOK at a human brain, almost everything you see is neocortex. The neocortex is a sheet about two to four millimeters thick. As the neocortex got bigger, the surface area of this sheet expanded. To fit in the skull, it became folded, the way you would bunch up a towel to fit it in a suitcase. If you unfolded a human neocortical sheet, it would be almost three square feet in surface area—about the size of a small desk.
Early experimentation led to the conclusion that the neocortex didn’t serve any one function and instead subserved a multitude of different functions. For example, the back of the neocortex processes visual input and hence is called the visual cortex.* If you removed your visual cortex, you would become blind. If you record the activity of neurons in the visual cortex, they respond to specific visual features at specific locations, such as certain colors or line orientations. If you stimulate neurons within the visual cortex, people will report seeing flashes of lights.
In a nearby region called the auditory cortex, the same thing occurs with auditory perception. Damage to one’s auditory cortex impairs one’s ability to perceive and understand sounds. If you record the activity of neurons in the auditory cortex, you’ll find they are responsive to specific frequencies of sound. If you stimulate certain neurons within the auditory cortex, people will report hearing noises.
Figure 11.1: The human neocortex
Original art by Rebecca Gelernter
There are other neocortical regions for touch, pain, and taste. And there are other areas of the neocortex that seem to serve even more disparate functions—there are areas for movement, language, and music.
At first glance, this makes no sense. How can one structure do so many different things?
Mountcastle’s Crazy Idea
In the mid-twentieth century, the neuroscientist Vernon Mountcastle was pioneering what was, at the time, a new research paradigm: recording the activity of individual neurons in the neocortex of awake-behaving animals. This new approach offered a novel view of the inner workings of brains as animals went about their life. He used electrodes to record the neurons in the somatosensory cortices (the neocortical area that processes touch input) of monkeys to see what types of touch
One of the first observations Mountcastle made was that neurons within a vertical column (about five hundred microns in diameter) of the neocortical sheet seemed to all respond similarly to sensory stimuli, while neurons horizontally farther away did not. For example, an individual column within the visual cortex might contain neurons that all similarly responded to bars of light at specific orientations at a specific location in the visual field. However, neurons within nearby columns responded only to bars of light at different orientations or locations. This same finding has been confirmed within multiple modalities. In rats, there are columns of neocortex that respond only to the touch of a specific single whisker, with each nearby column responding to a completely different whisker. In the auditory neocortex, there are individual columns that are selective for specific frequencies of sound.
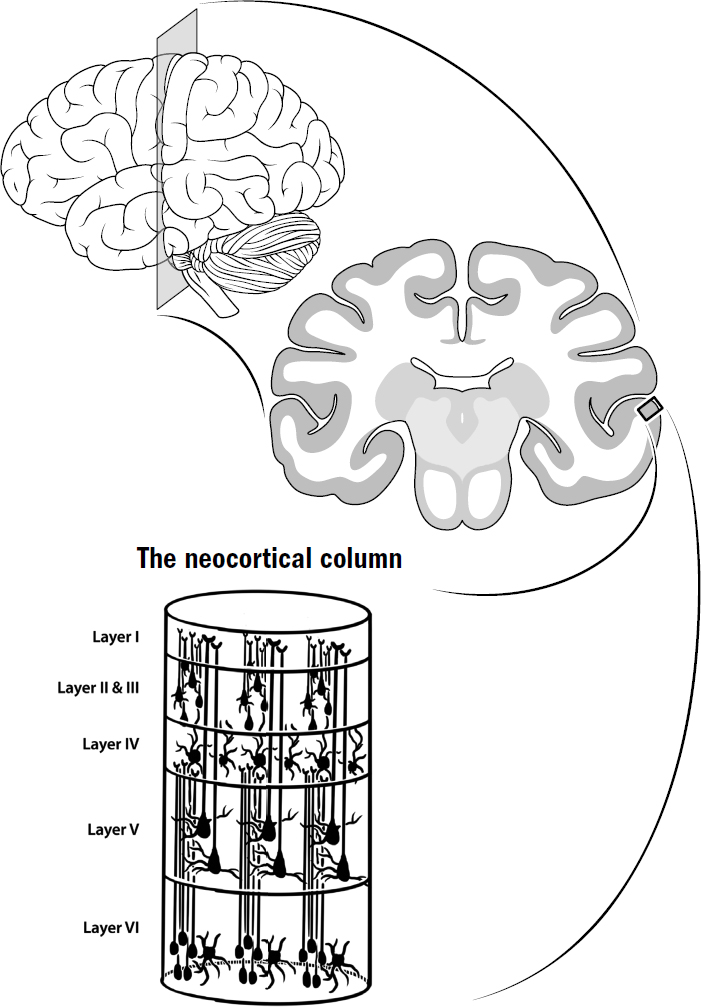
Figure 11.2: The neocortical column
Original art by Rebecca Gelernter
The second observation that Mountcastle made was that there were many connections vertically within a column and comparatively fewer connections between columns.
The third and final observation Mountcastle made was that under a microscope, the neocortex looked largely identical everywhere. The auditory neocortex, somatosensory neocortex, and visual neocortex all contain the same types of neurons organized in the same way. And this is true across species of mammals—the neocortex of a rat, a monkey, and a human all look relatively the same under a microscope.
These three facts—vertically aligned activity, vertically aligned connectivity, and observed similarity between all areas of neocortex—led Mountcastle to a remarkable conclusion: the neocortex was made up of a repeating and duplicated microcircuit, what he called the neocortical column. The cortical sheet was just a bunch of neocortical columns packed densely together.
This provided a surprising answer to the question of how one structure can do so many different things. According to Mountcastle, the neocortex does not do different things; each neocortical column does exactly the same thing. The only difference between regions of neocortex is the input they receive and where they send their output; the actual computations of the neocortex itself are identical. The only difference between, for example, the visual cortex and the auditory cortex is that the visual cortex gets input from the retina, and the auditory cortex gets input from the ear.
Remarkably, the ferrets could see just fine. And when researchers recorded the area of the neocortex that was typically auditory but was now receiving input from the eyes, they found the area responded to visual stimuli just as the visual cortex would. The auditory and visual cortices are interchangeable.
This was further reinforced by studies of congenitally blind patients whose retinas had never sent any signals to their brains. In these patients, the visual cortex never received input from the eyes. However, if you record the activity of neurons in the visual cortex of congenitally blind humans, you find that the visual cortex has not been rendered a functionally useless region. Instead, it becomes responsive to a multitude of other sensory input, such as sounds and touch. This puts meat on the bone of the idea that people who are blind do, in fact, have superior hearing—the visual cortex becomes repurposed to aid in audition. Again, areas of neocortex seem interchangeable.
Consider stroke patients. When patients have damage to a specific area of neocortex, they immediately lose the function in that area. If the motor cortex is damaged, patients can become paralyzed. If the visual cortex is damaged, patients become partially blind. But over time, function can return. This is usually not the consequence of the damaged area of neocortex recovering; typically, that area of neocortex remains dead forever. Instead, nearby areas of neocortex become repurposed to fulfill the functions of the now-damaged area of neocortex. This too suggests that areas of neocortex are interchangeable.
To those in the AI community, Mountcastle’s hypothesis is a scientific gift like no other. The human neocortex is made up of over ten billion neurons and trillions of connections; it is a hopeless endeavor to try and decode the algorithms and computations performed by such an astronomically massive hairball of neurons. So hopeless that many neuroscientists believe that attempting to decode how the neocortex works is a fruitless endeavor, doomed to fail. But Mountcastle’s theory offers a more hopeful research agenda—instead of trying to understand the entire human neocortex, perhaps we only have to understand the function of the microcircuit that is repeated a million or so times. Instead of understanding the trillions of connections in the entire neocortex, perhaps we only have to understand the million or so connections within the neocortical column. Further, if Mountcastle’s theory is correct, it suggests that the neocortical column implements some algorithm that is so general and universal that it can be applied to extremely diverse functions such as movement, language, and perception across every sensory modality.
The basics of this microcircuit can be seen under a microscope. The neocortex contains six layers of neurons (unlike the three-layered cortex seen in earlier vertebrates). These six layers of neurons are connected in a complicated but beautifully consistent way. There is a specific type of neuron in layer five that always projects to the basal ganglia, the thalamus, and the motor areas. In layer four, there are neurons that always get input directly from the thalamus. In layer six, there are neurons that always project to the thalamus. It is not just a soup of randomly connected neurons; the microcircuit is prewired in a specific way to perform some specific computation.
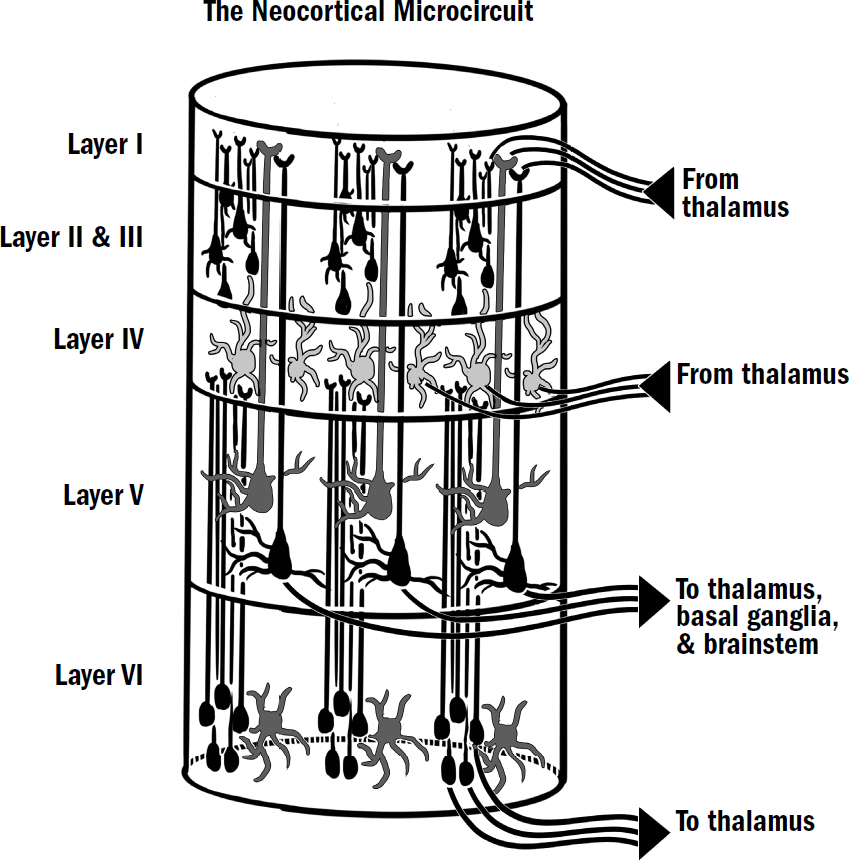
Figure 11.3: The microcircuitry of the neocortical column
Original art by Mesa Schumacher
The question is, of course: What is the computation?
Peculiar Properties of Perception
In the nineteenth century, the scientific study of human perception began in full force. Scientists around the world started probing the mind. How does vision work? How does audition work?
The inquiry into perception began with the use of illusions; by manipulating people’s visual perceptions, scientists uncovered three peculiar properties of perception. And because much of perception, in humans at least, occurs in the neocortex, these properties of perception teach us about how the neocortex works.
Property #1: Filling In
This filling in is not a property unique to vision; it is seen across most of our sensory modalities. This is how you can still understand what someone is saying through a garbled phone connection and how you can identify an object through touch even with your eyes closed.

“Editor” from Jastrow, 1899. Others from Lehar, 2003.
Property #2: One at a Time
Staircase from Schroeder, 1858. “Necker cube” from Necker, 1832. Duck or rabbit from from Jastrow, 1899.
What is interesting about all these ambiguous pictures is that your brain can see only one interpretation at a time. You cannot see a duck and a rabbit simultaneously, even though the sensory evidence is equally suggestive of both. The mechanisms of perception in the brain, for some reason, require it to pick only one.
This also applies to audition. Consider the “cocktail-party effect.” If you are at a noisy cocktail party, you can tune in to the conversation of the person you are speaking to or the conversation of a nearby group. But you cannot listen to both conversations at the same time. No matter which conversation you tune in to, the auditory input into your ear is identical; the only difference is what your brain infers from that input. You can perceive only a single conversation at a time.
Property #3: Can’t Unsee
What happens when sensory evidence is vague—when it isn’t clear that it can be interpreted as anything meaningful at all? Consider the image in 11.6. If you haven’t seen these before, they will look like nothing—just blobs. If I give you a reasonable interpretation of these blobs, all of a sudden, your perception of them will change.

Image from Fahle et al., 2002. Used with permission by The MIT Press.
In the nineteenth century, a German physicist and physician named Hermann von Helmholtz proposed a novel theory to explain these properties of perception. He suggested that a person doesn’t perceive what is experienced; instead, he or she perceives what the brain thinks is there—a process Helmholtz called inference. Put another way: you don’t perceive what you actually see, you perceive a simulated reality that you have inferred from what you see.
This idea explains all three of these peculiar properties of perception. Your brain fills in missing parts of objects because it is trying to decipher the truth that your vision is suggesting (“Is there actually a sphere there?”). You can see only one thing at a time because your brain must pick a single reality to simulate—in reality, the animal can’t be both a rabbit and a duck. And once you see that an image is best explained as a frog, your brain maintains this reality when observing it.
Generative Models: Recognizing by Simulating
In the 1990s, Geoffrey Hinton and some of his students (including the same Peter Dayan that had helped discover that dopamine responses are temporal difference learning signals) set their sights on building an AI system that learned in the way that Helmholtz suggested.

One class of unsupervised-learning methods are auto-associative networks, like those we speculated emerged in the cortex of early vertebrates. Based on correlations in input patterns, these networks cluster common patterns of input into ensembles of neurons, offering a way in which overlapping patterns can be recognized as distinct, and noisy and obstructed patterns can be completed.
But Helmholtz suggested that human perception was doing something more than this. He suggested that instead of simply clustering incoming input patterns based on their correlations, human perception might optimize for the accuracy with which the inner simulated reality predicts the current external sensory input.
In 1995, Hinton and Dayan came up with a proof of concept for Helmholtz’s idea of perception by inference; they named it
Hinton tested this network with images of handwritten numbers between 0 and 9. A picture of a handwritten number can be given at the bottom of the network (one neuron for each pixel) and will flow upward and activate a random set of neurons at the top. These activated neurons at the top can then flow back down and activate a set of neurons at the bottom to produce a picture of its own. Learning was designed to get the network to stabilize to a state where input that flows up the network is accurately re-created when it flows back down.
At first, there will be big discrepancies between the values in neurons from the image flowing in and the result flowing out. Hinton designed this network to learn with two separate modes: recognition mode and generative mode. When in recognition mode, information flows up the network (starting from an input picture of a 7 to some neurons at the top), and the backward weights are nudged to make the neurons activated at the top of the network better reproduce the input sensory data (make a good simulated 7). In contrast, when in generative mode, information flows down the network (starting from the goal to produce an imagined picture of a 7), and the forward weights are nudged so that the neurons activated at the bottom of the network are correctly recognized at the top (“I recognize what I just made as a 7”).
Nowhere was this network told the right answer; it was never told what properties make up a 2 or even which pictures were 2s or 7s or any other number. The only data the network had to learn from was pictures of numbers. The question was, of course, would this work? Would this toggling back and forth between recognition and generation enable the network to both recognize handwritten numbers and generate its own unique pictures of handwritten numbers without ever having been told the right answer?
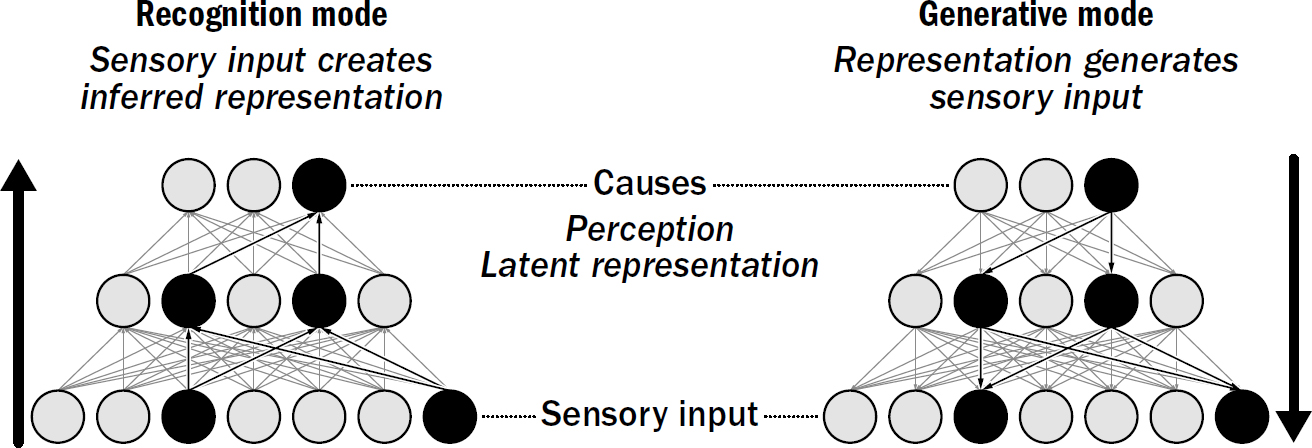
Figure 11.7: The Helmholtz Machine
Original art by Rebecca Gelernter
This might not seem particularly impressive. You gave a network a picture of a number, and it spit out a picture of that same number—what’s the big deal? There are three attributes of this network that are groundbreaking. First, the top of this network now reliably “recognizes” imperfectly handwritten letters without any supervision. Second, it generalizes impressively well; it can tell that two differently handwritten pictures of 7s are both a 7—they will activate a similar set of neurons at the top of the network. And third, and most important, this network can now generate novel pictures of handwritten numbers. By manipulating neurons at the top of this network, you can create lots of handwritten 7s or handwritten 4s or any number it has learned. This network has learned to recognize by generating its own data.

Image from Hinton et al., 1995. Used with permission.
The Helmholtz machine was an early proof of concept of a much broader class of models called generative models. Most modern generative models are more complicated than the Helmholtz machine, but they share the essential property that they learn to recognize things in the world by generating their own data and comparing the generated data to the actual data.

Figure 11.9: StyleGAN2 from thispersondoesnotexist.com
Pictures from thispersondoesnotexist.com
While most AI advancements that occurred in the early 2000s involved applications of supervised-learning models, many of the recent advancements have been applications of generative models. Deepfakes, AI-generated art, and language models like GPT-3 are all examples of generative models at work.
Helmholtz suggested that much of human perception is a process of inference—a process of using a generative model to match an inner simulation of the world to the sensory evidence presented. The success of modern generative models gives weight to his idea; these models reveal that something like this can work, at least in principle. It turns out that there is, in fact, an abundance of evidence that the neocortical microcircuit is implementing such a generative model.
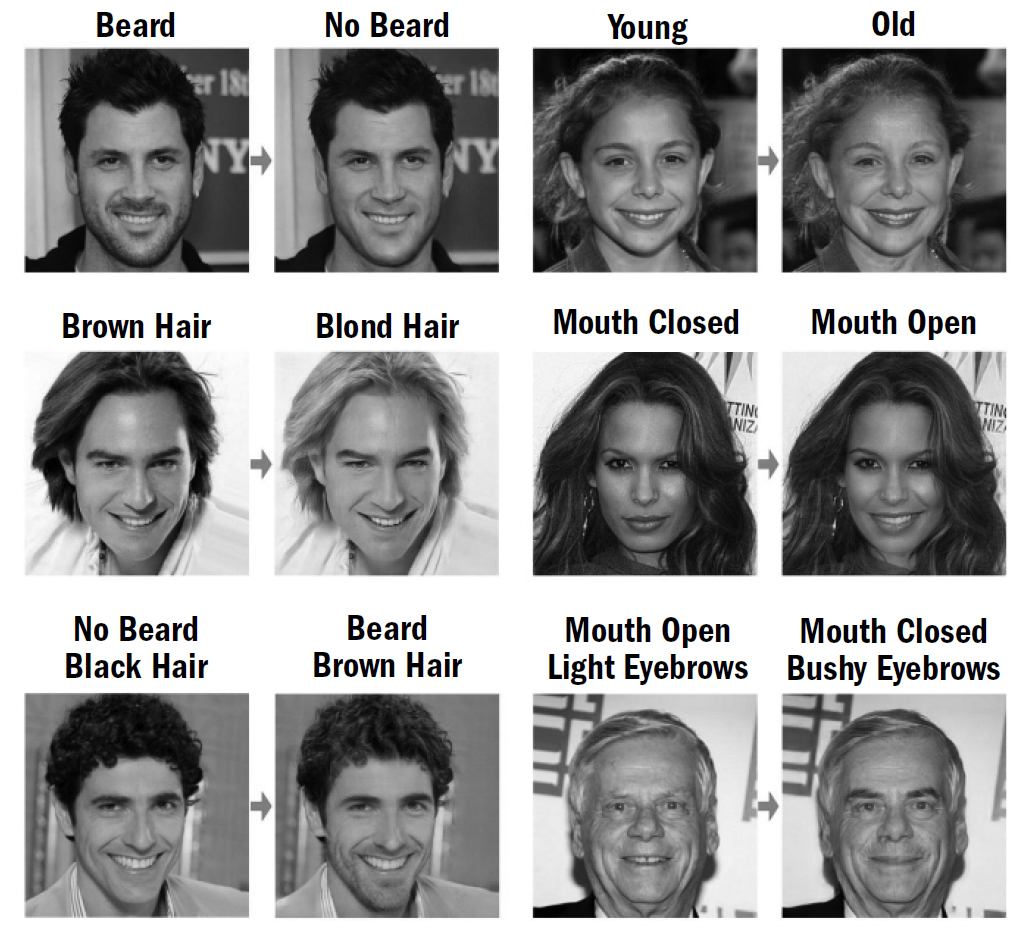
Figure from He et al., 2019. Used with permission.
Hallucinating, Dreaming, and Imagining: The Neocortex as a Generative Model
People whose eyes stop sending signals to their neocortex, whether due to optic-nerve damage or retinal damage, often get something called Charles Bonnet syndrome. You would think that when someone’s eyes are disconnected from their brain, they would no longer see. But the opposite happens—for several months after going blind, people start seeing a lot. They begin to hallucinate. This phenomenon is consistent with a generative model: cutting off sensory input to the neocortex makes it unstable. It gets stuck in a drifting generative process in which visual scenes are simulated without being constrained to actual sensory input—thus you hallucinate.
This idea of perception as a constrained hallucination is, of course, exactly what Helmholtz meant by inference and exactly what a generative model is doing. We match our inner hallucination of reality to the sensory data we are seeing. When the visual data suggests there is a triangle in a picture (even if there is not actually a triangle there), we hallucinate a triangle, hence the filling-in effect.
Many features of imagination in mammals are consistent with what we would expect from a generative model. It is easy, even natural, for humans to imagine things that they are not currently experiencing. You can imagine the dinner you ate last night or imagine what you will be doing later today. What are you doing when you are imagining something? This is just your neocortex in generation mode. You are invoking a simulated reality in your neocortex.
None of this is an obvious result. Imagination could have been performed by a system separate from recognition. But in the neocortex, this is not the case—they are performed in the exact same area. This is exactly what we would expect from a generative model: perception and imagination are not separate systems but two sides of the same coin.
Predicting Everything
One way to think about the generative model in the neocortex is that it renders a simulation of your environment so that it can predict things before they happen. The neocortex is continuously comparing the actual sensory data with the data predicted by its simulation. This is how you can immediately identify anything surprising that occurs in your surroundings.
As you walk down the street, you are not paying attention to the feelings of your feet. But with every movement you make, your neocortex is passively predicting what sensory outcome it expects. If you placed your left foot down and didn’t feel the ground, you would immediately look to see if you were about to fall down a pothole. Your neocortex is running a simulation of you walking, and if the simulation is consistent with sensor data, you don’t notice it, but if its predictions are wrong, you do.
Brains have been making predictions since early bilaterians, but over evolutionary time, these predictions became more sophisticated. Early bilaterians could learn that the activation of one neuron tended to precede the activation of another neuron and could thereby use the first neuron to predict the second. This was the simplest form of prediction. Early vertebrates could use patterns in the world to predict future rewards. This was a more sophisticated form of prediction. Early mammals, with the neocortex, learned to predict more than just the activation of reflexes or future rewards; they learned to predict everything.
The neocortex seems to be in a continuous state of predicting all its sensory data. If reflex circuits are reflex-prediction machines, and the critic in the basal ganglia is a reward-prediction machine, then the neocortex is a world-prediction machine—designed to reconstruct the entire three-dimensional world around an animal to predict exactly what will happen next as animals and things in their surrounding world move.
Somehow the neocortical microcircuit implements such a general system that it can render a simulation of many types of input. Give it visual input and it will learn to render a simulation of the visual aspects of the world; give it auditory input and it will learn to render a simulation of auditory aspects of the world. This is why the neocortex looks the same everywhere. Different subregions of neocortex simulate different aspects of the external world based on the input they receive. Put all these neocortical columns together, and they make a symphony of simulations that render a rich three-dimensional world filled with objects that can be seen, touched, and heard.
How the neocortex does this is still a mystery. At least one possibility is that it is prewired to make a set of clever assumptions. Modern AI models are often viewed as narrow—that is, they’re able to work in a narrow set of situations they are specifically trained for. The human brain is considered general—it is able to work in a broad set of situations. The research agenda has therefore been to try and make AI more general. However, we might have it backward. One of the reasons why the neocortex is so good at what it does may be that, in some ways, it is far less general than our current artificial neural networks. The neocortex may make explicit narrow assumptions about the world, and it may be exactly these assumptions that enable it to be so general.
The Evolution of Prediction
PREDICTION IN EARLY BILATERIANS | PREDICTION IN EARLY VERTEBRATES | PREDICTION IN EARLY MAMMALS |
Predict reflex activation | Predict future rewards | Predict all sensory data |
Reflex circuits | Cortex and basal ganglia | Neocortex |
This provides some intution about what Helmholtz meant by inference—the generative model in the neocortex tries to infer the causes of its sensory input. Causes are just the inner simulated 3D world that the neocortex believes best matches the sensory input it is being given. This is also why generative models are said to try to explain their input—your neocortex attempts to render a state of the world that could produce the picture that you are seeing (e.g., if a frog was there, it would “explain” why those shadows look the way they do).
But why do this? What is the point of rendering an inner simulation of the external world? What value did the neocortex offer these ancient mammals?
There are many ongoing debates about what is missing in modern AI systems and what it will take to get AI systems to exhibit human-level intelligence. Some believe the key missing pieces are language and logic. But others, like Yann LeCun, head of AI at Meta, believe they are something else, something more primitive, something that evolved much earlier. In LeCun’s words:
The simulation rendered in the neocortices of mammals (and perhaps in similar structures of birds or even octopuses) is exactly this missing “world model.” The reason the neocortex is so powerful is not only that it can match its inner simulation to sensory evidence (Helmholtz’s perception by inference) but, more important, that its simulation can be independently explored. If you have a rich enough inner model of the external world, you can explore that world in your mind and predict the consequences of actions you have never taken. Yes, your neocortex enables you to open your eyes and recognize the chair in front of you, but it also enables you to close your eyes and still see that chair in your mind’s eye. You can rotate and modify the chair in your mind, change its colors, change its materials. It is when the simulation in your neocortex becomes decoupled from the real external world around you—when it imagines things that are not there—that its power becomes most evident.
This was the gift the neocortex gave to early mammals. It was imagination—the ability to render future possibilities and relive past events—that was the third breakthrough in the evolution of human intelligence. From it emerged many familiar features of intelligence, some of which we have re-created and surpassed in AI systems, others of which are still beyond our grasp. But all of them evolved in the minuscule brains of the first mammals.
In the coming chapters, we will learn how the neocortex enabled early mammals to perform feats like planning, episodic memory, and causal reasoning. We will learn how these tricks were repurposed to enable fine motor skills. We will learn about how the neocortex implements attention, working memory, and self-control. We will see that it is in the neocortex of early mammals where we will find many of the secrets to human-like intelligence, those that are missing from even our smartest AI systems.
12
Mice in the Imaginarium
THE EMERGENCE OF the neocortex was a watershed moment in the evolutionary history of human intelligence. The original function of the neocortex was surely not as broad as its modern applications—it wasn’t for pondering the nature of existence, planning careers, or writing poetry. Instead, the first neocortex gifted early mammals something more foundational: the ability to imagine the world as it is not.
Most research on the neocortex has focused on its impressive ability to recognize objects: to see a single picture of a face and easily identify it at many scales, translations, and rotations. In the context of early generative models, the generative mode—the process of simulation—is often viewed as the means to achieving the benefit of recognition. In other words, recognition is what is useful; imagination is a byproduct. But the cortex that came before the neocortex could also recognize objects quite well; even fish can recognize objects when
The core evolutionary function of the neocortex might have been the opposite—recognition might have been the means that unlocked the adaptive benefit of simulating. This would suggest that the original evolutionary function of the neocortex was not recognizing the world—an ability the older vertebrate cortex already had—but instead imagining and simulating the world, an ability the older cortex was lacking.
There were three new abilities that neocortical simulating provided early mammals, all three of which were essential for surviving the one-hundred-and-fifty-million-year predatory onslaught of sharp-toothed dinosaurs.
New Ability #1: Vicarious Trial and Error
In the 1930s, the psychologist Edward Tolman, working at UC Berkeley, was putting rats in mazes to see how they learned. Normal psychology-type work at the time—this was the generation that followed Thorndike. The research paradigm of Thorndike’s law of effect, in which animals repeated behavior that had pleasant consequences, was in full force.
Tolman made a speculation: The rat was “playing out” each option before taking it. Tolman called this “vicarious trial and error.”
Of course, the fact that you can observe a rat pause and turn its head back and forth doesn’t prove that it is, in fact, imagining going down different paths. And due to this lack of evidence, the idea of vicarious trial and error faded out of favor in the decades that followed Tolman’s observation. It was only recently, in the 2000s, that technology reached the point where ensembles of neurons in the brain of a rat could be recorded in real time as rats navigated their environment. This was the first time that neuroscientists could literally watch what was happening in the brains of rats when they paused and toggled their heads back and forth.
It was David Redish and his student Adam Johnson, neuroscientists at the University of Minnesota, who first probed what was happening in the brain of rats during these choices. At the time, it was well known that when a rat navigates a maze, specific place cells in its hippocampus get activated. This was similar to the spatial map of a fish—specific hippocampal neurons encode specific locations. In a fish, these neurons become active only when the fish is physically present at the encoded location—but when Redish and Johnson recorded these neurons in rats, they found something different: when the rat stopped at the decision point and turned its head back and forth, its hippocampus ceased to encode the actual location of the rat and instead went back and forth rapidly playing out the sequence of place codes that made up both possible future paths from the choice point. Redish could literally see the rat imagining future paths.
How groundbreaking this was cannot be overstated—neuroscientists were peering directly into the brain of a rat, and directly observing the rat considering alternative futures. Tolman was right: the head toggling behavior he observed was indeed rats planning their future actions.
In contrast, the first vertebrates did not plan their actions ahead of time. We can see this by examining their cold-blooded descendants—modern fish and reptiles—who show no evidence of learning by vicarious trial and error.
Consider the detour task. Take a fish and put it in a tank with a transparent barrier in the middle of the tank. Put a small hole in one corner of the barrier so that the fish can get from one side to the other. Let the fish explore the tank, find the hole, and spend some time swimming back and forth. After several days, do something new: Put the fish on one side of the tank and put a treat on the opposite side of the transparent barrier. What happens?
Why is this? While the fish had swum through the hole to get to the other side of the tank before, it had never learned that the path through the hole provided dopamine. Trial-and-error learning had never trained the fish’s basal ganglia that when it saw food across the transparent barrier, it should take the action of swimming through the hole to get the food.
Another problem with the older strategy of learning by doing is that sometimes past rewards are not predictive of current rewards because an animal’s internal state has changed. For example, put a rat in a maze where one side provides overly salty food and the other side provides normal food. Let the rat navigate this maze normally and try the overly salty food (which it will hate and then avoid) and the normal food (which the rat will enjoy). Now suppose you put the rat back in that situation but with a twist: You make it severely salt-deprived. What does the rat do?
New Ability #2: Counterfactual Learning
Humans spend a painful amount of time wallowing in regret. Questions you might hear from an average human conversation: “What would life have been like if I had said yes when Ramirez offered to throw our lives away and move to Chile to work on his farm?” “What if I had followed my dream and pursued baseball instead of this desk job?” “Why did I say that stupid thing at work today? What would have happened had I said something cleverer?”
Buddhists and psychologists alike realize that ruminating about what could have been is a source of great misery for humanity. We cannot change the past, so why torture ourselves with it? The evolutionary roots of this go back to early mammals. In the ancient world, and in much of the world that followed, such ruminating was useful because often the same situation would recur and a better choice could be made.
The type of reinforcement learning we saw in early vertebrates has a flaw: It can only reinforce the specific action actually taken. The problem with this strategy is that the paths that were actually taken are a small subset of all the possible paths that could have been taken. What are the chances that an animal’s first attempt picked the best path?
If a fish swims into a shoal to hunt some invertebrates and comes away with one, and a nearby fellow fish took a different path and came away with four, the first fish won’t learn from that mistake; it will merely reinforce the path taken with the mediocre reward. What fish are missing is the ability to learn from counterfactuals. A counterfactual is what the world would be now if you had made a different choice in the past.
Figure 12.1: Redish restaurant-row test of regret in rats
Original figure by Max Bennett, with oversight and permission from David Redish
Consider the choice presented to the rats at a given corridor: Do I wait here for mediocre banana that the tone just signaled would be released in five seconds, or do I run to the next door, which contains my favorite food, cherry, and gamble that it will also be released quickly? When rats chose to forgo quick access to a banana treat to try the cherry door and the next tone signaled a long wait of forty-five seconds, rats showed all the signs of regretting their choice. They paused and looked back toward the corridor that they had passed and could no longer go back to. And the neurons in the taste area of the neocortex reactivated the representation of banana, showing that rats were literally imagining a world where they had made a different choice and got to eat the banana.
The rats that turned back and reactivated the representation of the forgone choice also ended up changing their future choices. They waited longer the next time and ate other food more hastily to try and get back around the maze to the cherry to try again.
Counterfactual learning represented a major advancement in how ancestral brains solved the credit assignment problem. As a reminder, the credit assignment problem is this: When some important event occurs that you want to be able to predict ahead of time, how do you choose what previous actions or events to give credit for having been predictive of the event? In simple terms: A bunch of things happen (a bird chirps, a gust of wind blows, a leaf moves, and lightning strikes), and then a fire appears—what do you give credit for being a good predictor of fire? In early bilaterians, simple tricks like blocking, latent inhibition, and overshadowing drove the logic by which simple predictions and associations were made. In early vertebrates, the evolution of temporal difference learning enabled the basal ganglia to assign credit using changes in future predicted reward; when the critic thinks the situation just got better or worse is when cues or actions are given credit. But in early mammals, with their ability to simulate alternative pasts, credit could also be assigned with the counterfactual. By asking “Would I have lost the game had I not made this move?,” mammals can determine whether a move truly deserves credit for winning the game.
Causation itself may live more in psychology than in physics. There is no experiment that can definitively prove the presence of causality; it is entirely immeasurable. Controlled experiments we run may suggest causation, but they always fall short of proof because you can, in fact, never run a perfectly controlled experiment. Causation, even if real, is always empirically out of reach. In fact, modern experiments in the field of quantum mechanics suggest that causation may not even exist, at least not everywhere. The laws of physics may contain rules of how features of reality progress from one time step to the next without any real causal relationships between things at all. Ultimately, whether causality is real or not, the evolution of our intuitive perception of causality does not derive from its reality but from its usefulness. Causation is constructed by our brains to enable us to learn vicariously from alternative past choices.
The Evolution of Credit Assignment
CREDIT ASSIGNMENT IN EARLY BILATERIANS | CREDIT ASSIGNMENT IN EARLY VERTEBRATES | CREDIT ASSIGNMENT IN EARLY MAMMALS |
Credit assigned based on basic rules of blocking, latent inhibition, and overshadowing | Credit assigned based on when the critic predicts changes in future rewards | Credit assigned based on the counterfactual–which previous events or actions, if they had not occurred, would have prevented the subsequent event from occurring (i.e., what truly caused the event?) |
New Ability #3: Episodic Memory
In September 1953, a twenty-seven year old man named Henry Molaison underwent an experimental procedure that removed his entire hippocampus—the source of his debilitating seizures. The surgery was, in one sense, a success: severity of his seizures were markedly reduced and he retained his personality and intellect. But his doctors quickly discovered that the surgery had deprived their patient of something precious: upon waking up, Molaison was entirely unable to produce new memories. He could hold a conversation for a minute or two, but shortly thereafter would forget everything that just happened. Even forty years later, he could fill out crosswords with facts from before 1953, but not with facts that occurred after. Molaison was stuck in 1953.
We don’t review the past only for the purpose of considering alternative past choices; we also review the past to remember previous life events. People can easily recall what they did five minutes ago or what they majored in at college or that funny joke made during a wedding speech. This form of memory, in which we recall specific past episodes of our lives, is called “episodic memory.” This is distinct from, say, procedural memory, where we remember how to do various movements, such as speaking, typing, or throwing a baseball.
But here is the weird thing—we don’t truly remember episodic events. The process of episodic remembering is one of simulating an approximate re-creation of the past. When imagining future events, you are simulating a future reality; when remembering past events, you are simulating a past reality. Both are simulations.
After Molaison’s surgery, he became the most studied neuroscience patient in history: why was the hippocampus required for creating new episodic memories, but not for retrieving old ones? This is an example of evolution repurposing old structures for new purposes. In mammal brains, episodic memory emerges from a partnership between the older hippocampus and the newer neocortex. The hippocampus can quickly learn patterns, but cannot render a simulation of the world; the neocortex can simulate detailed aspects of the world, but cannot learn new patterns quickly. Episodic memories must be stored rapidly, and thus the hippocampus, designed for the rapid pattern recognition of places, was repurposed to also aid in the rapid encoding of episodic memories. Distributed neural activations of sensory neocortex (i.e., simulations) can be “retrieved” by reactivating the corresponding pattern in the hippocampus. Just as rats reactivated place cells in the hippocampus to simulate going down different paths, rats can reactivate these “memory codes” in the hippocampus to rerender simulations of recent events.
MODEL-FREE REINFORCEMENT LEARNING | MODEL-BASED REINFORCEMENT LEARNING |
Learns direct associations between a current state and the best actions | Learns a model of how actions affect the world and uses this to simulate different actions before choosing |
Faster decisions but less flexible | Slower decisions but more flexible |
Emerged in early vertebrates | Emerged in early mammals |
Neocortex is not required | Neocortex is required |
Example: Habitually going to work by just responding to each cue (traffic light, landmark) as it comes up | Example: Considering different ways to get to work and picking the one that got you there the fastest in your mind. |
This dynamic provided a new solution to the catastrophic forgetting problem, whereby neural networks forget old patterns when they learn new ones. By retrieving and replaying recent memories alongside old memories, the hippocampus aids the neocortex in incorporating new memories without disrupting old ones. In AI, this process is called “generative replay” or “experience replay” and has been shown to be an effective solution to catastrophic forgetting. This is why the hippocampus is necessary for creating new memories, but not for retrieving old ones; the neocortex can retrieve memories on its own after a sufficient amount of replay.
There is another category of reinforcement learning called model-based reinforcement learning. These systems must learn something more complicated: a model of how their actions affect the world. Once such a model is constructed, these systems then play out sequences of possible actions before making choices. These systems are more flexible but are burdened with the difficult task of building and exploring an inner world model when making decisions.
Most of the reinforcement learning models employed in modern technology are model-free. The famous algorithms that mastered various Atari
Model-based reinforcement learning has proven to be more difficult to implement for two reasons.
First, building a model of the world is hard—the world is complex and the information we get about it is noisy and incomplete. This is LeCun’s missing world model that the neocortex somehow renders. Without a world model, it is impossible to simulate actions and predict their consequences.
The second reason model-based reinforcement learning is hard is that choosing what to simulate is hard. In the same paper that Marvin Minsky identified the temporal credit assignment problem as an impediment to artificial intelligence, he also identified what he called the “search problem”: In most real-world situations, it is impossible to search through all possible options. Consider chess. Building a world model of the game of chess is relatively trivial (the rules are deterministic, you know all the pieces, all their moves, and all the squares of the board). But in chess, you cannot search through all the possible future moves; the tree of possibilities in chess has more branching paths than there are atoms in the universe. So the problem is not just constructing an inner model of the external world but also figuring out how to explore it.
And yet clearly, somehow, the brains of early mammals solved the search problem. Let’s see how.
13
Model-Based Reinforcement Learning
AFTER THE SUCCESS of TD-Gammon, people tried to apply Sutton’s temporal difference learning (a type of model-free reinforcement learning) to more complex board games like chess. The results were disappointing.
While model-free approaches like temporal difference learning can do well in backgammon and certain video games, they do not perform well in more
How did AlphaZero achieve superhuman performance at Go and chess? How did AlphaZero succeed where temporal difference learning could not? The key difference was that AlphaZero simulated future possibilities. Like TD-Gammon, AlphaZero was a reinforcement learning system—its strategies were not programmed into it with expert rules but learned through trial and error. But unlike TD-Gammon, AlphaZero was a model-based reinforcement learning algorithm; AlphaZero searched through possible future moves before deciding what to do next.

Picture from https://en.wikipedia.org/wiki/Go_(game)#/media/File:FloorGoban.JPG
After its opponent moved, AlphaZero would pause, select moves to consider, and then play out thousands of simulations of how the entire game might go given those selected moves. After running a set of simulations, AlphaZero might see that it won thirty-five out of the forty imagined games when it made move A, thirty-nine of the forty imagined games when it made move B, and so on for many other possible next moves. AlphaZero could then pick the move where it had won the highest ratio of imagined games.
There are many algorithms for deciding how to prioritize which branches to search through in a large tree of possibilities. Google Maps uses such an algorithm when it searches for the optimal route from point A to point B. But the search strategy used by AlphaZero was different and offered unique insight into how real brains might work.
We already discussed how in temporal difference learning an actor learns to predict the best next move based on a hunch about the board position, doing so without any planning. AlphaZero simply expanded on this architecture. Instead of picking the single move its actor believed was the best next move, it picked multiple top moves that its actor believed were the best. Instead of just assuming its actor was correct (which it would not always be), AlphaZero used search to verify the actor’s hunches. AlphaZero was effectively saying to the actor, “Okay, if you think move A is the best move, let’s see how the game would play out if we did move A.” And AlphaZero then also explored other hunches of the actor, considering the second and third best moves the actor was suggesting (saying to the actor, “Okay, but if you didn’t take move A, what would your next best hunch be? Maybe move B will turn out even better than you think”).
What is elegant about this is that AlphaZero was, in some sense, just a clever elaboration on Sutton’s temporal difference learning, not a reinvention of it. It used search not to logically consider all future possibilities (something that is impossible in most situations) but to simply verify and expand on the hunches that an actor-critic system was already producing. We will see that this approach, in principle, may have parallels to how mammals navigate the search problem.
However, the most critical advantage of planning in mammalian brains over modern AI systems like AlphaZero is not their ability to plan with continuous action spaces, incomplete information, or complex rewards, but instead simply the mammalian brain’s ability to flexibly change its approach to planning depending on the situation. AlphaZero—which applied only to board games—employed the same search strategy with every move. In the real world, however, different situations call for different strategies. The brilliance of simulation in mammal brains is unlikely to be some special yet-to-be-discovered search algorithm; it is more likely to be the flexibility with which mammal brains employ different strategies. Sometimes we pause to simulate our options, but sometimes we don’t simulate things at all and just act instinctually (somehow brains intelligently decide when to do each). Sometimes we pause to consider possible futures, but other times we pause to simulate some past event or alternative past choices (somehow brains select when to do each). Sometimes we imagine rich details in our plans, playing out each individual detailed subtask, and sometimes we render just the general idea of the plan (somehow brains intelligently select the right granularity of our simulation). How do our brains do this?
Prefrontal Cortex and Controlling the Inner Simulation
In the 1980s, a neuroscientist named Antonio Damasio visited one of his patients—referred to as “L”—who had suffered a stroke. L lay in bed with her eyes open and a blank expression on her face. She was motionless and speechless, but she wasn’t paralyzed. She would, at times, lift up the blanket to cover herself with perfectly fine motor dexterity; she would look over at a moving object, and she could clearly recognize when someone spoke her name. But she did and said nothing. When looking into her eyes, people said it seemed that “she was there but not there.”
Figure 13.2
Original art by Mesa Schumacher
The frontal neocortex of a human brain contains three main subregions: motor cortex, granular prefrontal cortex (gPFC), and agranular prefrontal cortex (aPFC). The words granular and agranular differentiate parts of the prefrontal cortex based on the presence of granule cells, which are found in layer four of the neocortex. In the granular prefrontal cortex, the neocortex contains the normal six layers of neurons. However, in the agranular prefrontal cortex, the fourth layer of neocortex (where granule cells are found) is weirdly missing.* Thus, the parts of the prefrontal cortex that are missing layer four are called the agranular prefrontal cortex (aPFC), and the parts of the prefrontal cortex that contain a layer four are called the granular prefrontal cortex (gPFC). It is still unknown why, exactly, some areas of frontal cortex are missing an entire layer of neocortex, but we will explore some possibilities in the coming chapters.
The granular prefrontal cortex evolved much later in early primates, and we will learn all about it in breakthrough #4. The motor cortex evolved after the first mammals but before the first primates (we will learn about the motor cortex in the next chapter). But the agranular prefrontal cortex (aPFC) is the most ancient of frontal regions and evolved in the first mammals. It was the aPFC that was damaged in Damasio’s patient L. The aPFC is so ancient and fundamental to the proper functioning of the neocortex that when damaged, L was deprived of something central to what it means to be human—or, more specifically, what it means
In the first mammals, the entire frontal cortex was just agranular prefrontal cortex. All modern mammals contain an agranular prefrontal cortex inherited from the first mammals. To understand L’s akinetic mutism and how mammals decide when and what to simulate, we must first roll back the evolutionary clock to explore the function of the aPFC in the brains of the first mammals.
Figure 13.3: The frontal regions of the first mammals and of modern humans
Original art by Mesa Schumacher
While the frontal and sensory cortices seem to serve different functions (the frontal neocortex triggers simulations, the sensory neocortex renders simulations), they are both different areas of the neocortex and thus should execute the same fundamental computation. This presents a conundrum: How does the frontal neocortex, simply another area of neocortex, do something so seemingly different from the sensory neocortex? Why does a modern human with a damaged aPFC become devoid of intention? How does the aPFC trigger simulations in the sensory neocortex? How does it decide when to simulate something? How does it decide what to simulate?
Predicting Oneself
In a column of the sensory cortex, the primary input comes from external sensors, such as the eyes, ears, and skin. The primary input to the agranular prefrontal cortex, however, comes from the hippocampus, hypothalamus, and amygdala. This suggests that the aPFC treats sequences of places, valence activations, and internal affective states the way the sensory neocortex treats sequences of sensory information. Perhaps, then, the aPFC tries to explain and predict an animal’s own behavior the same way that the sensory neocortex tries to explain and predict the flow of external sensory information?
Perhaps the aPFC is always observing a rat’s basal-ganglia-driven choices and asking, “Why did the basal ganglia choose this?” The aPFC of a given rat might thereby learn, for example, that when the rat wakes up and has these specific hypothalamic activations, it always runs down to the river and consumes water. The aPFC might then learn that the why of such behavior is “to get water.” Then in similar circumstances, the aPFC can predict what the animal will do before the basal ganglia triggers any behavior—it can predict that when thirsty, the animal will run toward nearby water. In other words, the aPFC learns to model the animal itself, inferring the intent of behavior it observes, and uses this intent to predict what the animal will do next.
As philosophically fuzzy as intent might sound, it is conceptually no different from how the sensory cortex constructs explanations of sensory information. When you see a visual illusion that suggests a triangle (even when there is no triangle), your sensory neocortex constructs an explanation of it, which is what you perceive—a triangle. This explanation—the triangle—is not real; it is constructed. It is a computational trick that the sensory neocortex uses to make predictions. The explanation of the triangle enables your sensory cortex to predict what would happen if you reached out to grab it or turned the light on or tried to look at it from another angle.
Frontal vs Sensory Neocortex in the First Mammals
FRONTAL NEOCORTEX | SENSORY NEOCORTEX |
A self model | A world model |
Gets input from hippocampus, amygala, and hypothalamus | Gets input from sensory organs |
“I did this because I want to get to water” | “I see this because there is a triangle right there” |
Tries to predict what the animal will do next | Tries to predict what external objects will do next |
What is the evolutionary usefulness of this model of self in the frontal cortex? Why try to “explain” one’s own behavior by constructing “intent”? It turns out, this might be how mammals choose when to simulate things and how to select what to simulate. Explaining one’s own behavior might solve the search problem. Let’s see how.
How Mammals Make Choices
Let’s take the example of a rat navigating a maze and making a choice as to which direction to go when it reaches a fork. Going to the left leads to water, going to the right leads to food. It is these situations when vicarious trial and error occurs, and it occurs in three steps.
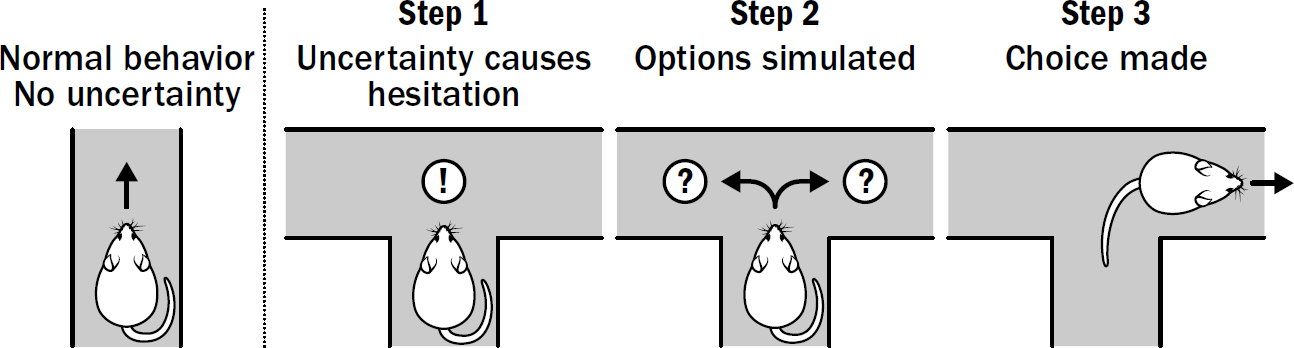
Figure 13.4: Speculations on how mammals make deliberative choices
Original art by Rebecca Gelernter
Step #1: Triggering Simulation
Either way, this provides a speculation for how mammal brains tackle the challenge of deciding when to go through the effort of simulating things. If events are unfolding as one would expect, there is no reason to waste time and energy simulating options, and it is easier just to let the basal ganglia drive decisions (model-free learning), but when uncertainty emerges (something new appears, some contingency is broken, or costs are close to the benefits), then simulation is triggered.
Step #2: Simulating Options
Okay, so the rat paused and decided to use simulation to resolve its uncertainty—now what? This brings us back to the search problem. A rat in a maze could do any one of a billion things, so how does it decide what to simulate?
We saw how AlphaZero solved this problem: It played out the top moves it was already predicting were the best. This idea aligns quite nicely with what is known about neocortical columns and the basal ganglia. The aPFC doesn’t sit there combing through every possible action, instead it specifically explores the paths that it is already predicting an animal will take. In other words, the aPFC searches through the specific options that different columns of the aPFC are already predicting. One set of columns predicted going left all the way to water, and another predicted going right, so there are only two different simulations to run.
After an animal pauses, different columns in the aPFC take turns playing out their predictions of what they think the animal will do. One group of columns plays out going left and following that path all the way to water. Another group of columns plays out going right and following that path all the way to get food.
Alternatively, it could be the basal ganglia that determines the actions taken during these simulations. This would be even closer to how AlphaZero worked—it selected simulated actions based on the actions its model-free actor predicted were best. In this case, it would be the aPFC that selects which of the divergent action predictions of the basal ganglia to simulate, but the basal ganglia would continue to decide which actions it wants to take in the imagined world rendered by the sensory neocortex.
Step #3: Choosing an Option
And so, as the process of vicarious trial and error unfolds, the results of these vicarious replays of behavior accumulate votes for each choice in the basal ganglia—the same way it would if the trial and error were not vicarious but real. If the basal ganglia keeps getting more excited by imagining drinking water than by imagining eating food (as measured by the amount of dopamine released), then these votes for water will quickly pass the choice threshold. The basal ganglia will take over behavior, and the rat will go get water.
The emergent effect of all this is that the aPFC vicariously trained the basal ganglia that left was the better option. The basal ganglia doesn’t know whether the sensory neocortex is simulating the current world or an imagined world. All the basal ganglia knows is that when it turned left, it got reinforced. Hence, when the sensory neocortex goes back to simulating the current world at the beginning of the maze, the basal ganglia immediately tries to repeat the behavior that was just vicariously reinforced. Voilà—the animal runs to the left to get water.
Goals and Habits (or the Inner Duality of Mammals)
In the early 1980s, a Cambridge psychologist by the name of Tony Dickinson was engaging in the popular psychology experiments of the time: training animals to push levers to get rewards. Dickinson was asking a seemingly mundane question: What happens if you devalue the reward of a behavior after the behavior is learned? Suppose you teach a rat that pushing a lever releases a food pellet from a nearby contraption. The rat will rapidly go back and forth between pushing the lever and gobbling up the food. Now suppose one day, completely out of the context of the box with the lever, you give the rat the same pellet and secretly lace it with a chemical that makes the rat feel sick. How does this change their behavior?
Dickinson had discovered habits. By engaging in the behavior five hundred times, rats had developed an automated motor response that was triggered by a sensory cue and completely detached from the higher-level goal of the behavior. The basal ganglia took over behavior without the aPFC pausing to consider what future these actions would produce. The behavior had been repeated so many times that the aPFC and basal ganglia did not detect any uncertainty and therefore the animal did not pause to consider the consequences.
Perhaps this is a familiar experience. People wake up and look at their phones without asking themselves why they are choosing to look at their phones. They keep scrolling through Instagram even though if someone had asked them if they wanted to keep scrolling, they’d say “no.” Of course, not all habits are bad: You don’t think about walking, and yet you walk perfectly; you don’t think about typing, and yet the thoughts flow effortlessly from your mind to your fingertips; you don’t think about speaking, and yet thoughts magically convert themselves into a repertoire of tongue, mouth, and throat movements.
Habits are automated actions triggered by stimuli directly (they are model-free). They are behaviors controlled directly by the basal ganglia. They are the way mammalian brains save time and energy, avoiding unnecessarily engaging in simulation and planning. When such automation occurs at the right times, it enables us to complete complex behaviors easily; when it occurs at the wrong times, we make bad choices.
The duality between model-based and model-free decision-making methods shows up in different forms across different fields. In AI, the terms model-based and model-free are used. In animal psychology, this same duality is described as goal-driven behavior and habitual behavior. And in behavioral economics, as in Daniel Kahneman’s famous book Thinking, Fast and Slow, this same duality is described as “system 2” (thinking slow) versus “system 1” (thinking fast). In all these cases, the duality is the same: Humans and, indeed, all mammals (and some other animals that independently evolved simulation) sometimes pause to simulate their options (model-based, goal-driven, system 2) and sometimes act automatically (model-free, habitual, system 1). Neither is better; each has its benefits and costs. Brains attempt to intelligently select when to do each, but brains do not always make this decision correctly, and this is the origin of many of our irrational behaviors.
The language used in animal psychology is revealing—one type of behavior is goal-driven and the other is not. Indeed, goals themselves may not have evolved until early mammals.
The Evolution of the First Goal
Just as the explanations of sensory information are not real (i.e., you don’t perceive what you see), so intent is not real; rather, it is a computational trick for making predictions about what an animal will do next.
This is important: The basal ganglia has no intent or goals. A model-free reinforcement learning system like the basal ganglia is intent-free; it is a system that simply learns to repeat behaviors that have previously been reinforced. This is not to say that such model-free systems are dumb or devoid of motivation; they can be incredibly intelligent and clever, and they can rapidly learn to produce behavior that maximizes the amount of reward. But these model-free systems do not have “goals” in the sense that they do not set out to pursue a specific outcome. This is one reason why model-free reinforcement learning systems are painfully hard to interpret—when we ask, “Why did the AI system do that?,” we are asking a question to which there is really no answer. Or at least, the answer will always be the same: because it thought that was the choice with the most predicted reward.
In contrast, the aPFC does have explicit goals—it wants to go to the fridge to eat strawberries or go to the water fountain to drink water. By simulating a future that terminates at some end result, the aPFC has an end state (a goal) that it seeks to achieve. This is why it is possible, at least in circumstances where people make aPFC-driven (goal-oriented, model-based, system 2) choices, to ask why a person did something.
When you pause and simulate different dinner options, choose to get pasta, then begin the long action sequence to get to the restaurant, this is a “volitional” choice—you can answer why you are getting in the car; you know the end state you are pursuing. In contrast, when you act only from habit, you have no answer as to why you did what you did.
Of course, the aPFC isn’t evolutionarily programmed to understand the goals of the animal, instead it learns these goals by first modeling behavior originally controlled by the basal ganglia. The aPFC constructs goals by observing behavior that is originally entirely devoid of them. And only once these goals are learned does the aPFC begin to exert control over behavior: the basal ganglia begins as the teacher of the aPFC, but as a mammal develops, these roles flip, and the aPFC becomes the teacher of the basal ganglia. And indeed, during brain development, agranular parts of the frontal cortex begin with a layer four that then slowly atrophies and disappears during development, leaving layer four largely empty. Perhaps this is part of a developmental program for constructing a model of self, starting by matching one’s internal model to its observations (hence beginning with a layer 4), and then transitioning to pushing behavior to match one’s internal model (hence no need for a layer 4 anymore). Again we see a beautiful bootstrapping in evolution.
This also offers some insight into the experience of Damasio’s patient L. It makes some sense why her head was “empty”: She was unable to render an inner simulation. She had no thoughts. She had no will to respond to anything because her inner model of intent was gone, and without that, her mind could not set even the simplest of goals. And without goals, tragically, nothing mattered.
How Mammals Control Themselves: Attention, Working Memory, and Self-Control
In a typical neuroscience textbook, the four functions ascribed to the frontal neocortex are attention, working memory, executive control, and, as we have already seen, planning. The connecting theme of these functions has always been confusing; it seems odd that one structure would subserve all these distinct roles. But through the lens of evolution, it makes sense that these functions are all intimately related—they are all different applications of controlling the neocortical simulation.
What is the point of attention? When a mouse selects an action sequence after its imagined simulation, it must stick to its plan as it runs down its path. This is harder than it sounds. The imagined simulation will not have been perfect; the mouse will not have predicted each sight, smell, and contour of the environment that it will actually experience. This means that the vicarious learning that the basal ganglia experienced will differ from the actual experience as the plan unfolds, and therefore, the basal ganglia may not correctly fulfill the intended behavior.
One way the aPFC can solve this problem is using attention. Suppose a rat’s basal ganglia learned, through trial and error, to run away from ducks and run toward rabbits. In this case, the basal ganglia will have opposite reactions to seeing the duck/rabbit depending on what pattern gets sent to it from the neocortex. If the aPFC had previously imagined seeing a rabbit and running toward it, then it can control the basal ganglia’s choices by using attention to ensure that when the rat sees this ambiguous picture, it sees a rabbit, not a duck.
Controlling ongoing behavior often also requires working memory—the maintenance of representations in the absence of any sensory cues. Many imagined paths and tasks involve waiting. For example, when a rodent forages among trees for nuts, it must remember which trees it has already foraged. This is a task shown to require the aPFC. If you inhibit a rodent’s aPFC during these delay periods, rodents lose their ability to perform such tasks from memory. And during such tasks, the aPFC exhibits “delay activity,” remaining activated even in the absence of any external cues. These tasks require the aPFC because working memory functions in the same way as attention and planning—it is the invoking of an inner simulation. Working memory—holding something in your head—is just your aPFC trying to keep re-invoking an inner simulation until you no longer need it.
In addition to planning, attention, and working memory, the aPFC can also control ongoing behavior more directly: It can inhibit the amygdala. There is a projection from the aPFC to inhibitory neurons surrounding the amygdala. During the fulfillment of an imagined plan, the aPFC can attempt to prevent the amygdala from triggering its own approach and avoidance responses. This was the evolutionary beginning of what psychologists call behavioral inhibition, willpower, and self-control: the persistent tension between our moment-to-moment cravings (as controlled by the amygdala and basal ganglia) and what we know to be a better choice (as controlled by the aPFC). In moments of willpower, you can inhibit your amygdala-driven cravings. In moments of weakness, the amygdala wins. This is why people become more impulsive when tired or stressed—the aPFC is energetically expensive to run, so if you are tired or stressed, the aPFC will be much less effective at inhibiting the amygdala.
To summarize: Planning, attention, and working memory are all controlled by the aPFC because all three are, in principle, the same thing. They are all different manifestations of brains trying to select what simulation to render. How does the aPFC “control” behavior? The idea presented here is that it doesn’t control behavior per se; it tries to convince the basal ganglia of the right choice by vicariously showing it that one choice is better and by filtering what information makes it to the basal ganglia. The aPFC controls behavior not by telling but by showing.
Early mammals had the ability to vicariously explore their inner model of the world, make choices based on imagined outcomes, and stick to the imagined plan once chosen. They could flexibly determine when to simulate futures and when to use habits; and they intelligently selected what to simulate, overcoming the search problem. They were our first ancestors to have goals.
14
The Secret to Dishwashing Robots
IMAGINE THE FOLLOWING. As you are holding this book your right hand begins to cramp up. The specific placement of each individual finger that you have effortlessly configured to perfectly balance the book in your hand begins to wilt as you lose control of the muscles in your right arm. You realize you can no longer control each finger individually; you can only open or close your hand with all your fingers moving at once, your hand transforming from a dexterous tool to an uncoordinated claw. Within minutes you can no longer even grasp the book with your right hand at all, and your arm becomes too weak to lift. This is what the experience of a stroke—the loss of blood flow to a region of the brain—feels like when it occurs in the motor cortex. Such a condition deprives its patients of fine motor skills and can even cause paralysis.
The motor cortex is a thin band of neocortex on the edge of the frontal cortex. Motor cortex makes up a map of the entire body, with each area controlling movements of specific muscles. While the entire motor cortex accounts for every part of the body, it does not dedicate equal space to each body part. Instead, it dedicates lots of space to the parts of the body that animals have skilled motor control over (in primates, this is the mouth and hands) and much less space to areas that they can’t control well (like the feet). This map in the motor cortex is mirrored in the adjacent somatosensory cortex—the region of the neocortex that processes somatosensory information (such as touch sensors in the skin and proprioceptive signals from muscles).
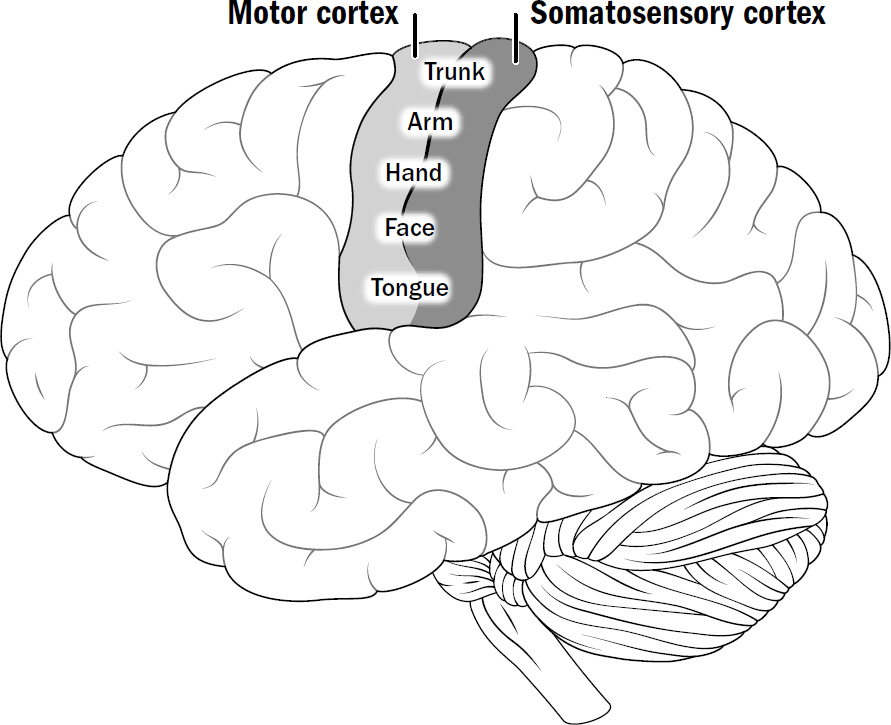
Figure 14.1: The motor cortex of humans
Original art by Rebecca Gelernter
In humans, the motor cortex is the primary system for controlling movement. Not only does stimulating specific areas of the motor cortex elicit movement from the corresponding body part, but damaging those same areas of the motor cortex creates paralysis in that same body part. The movement deficits of stroke patients almost always derive from damage to areas of the motor cortex. In chimpanzees, macaques, and lemurs, motor
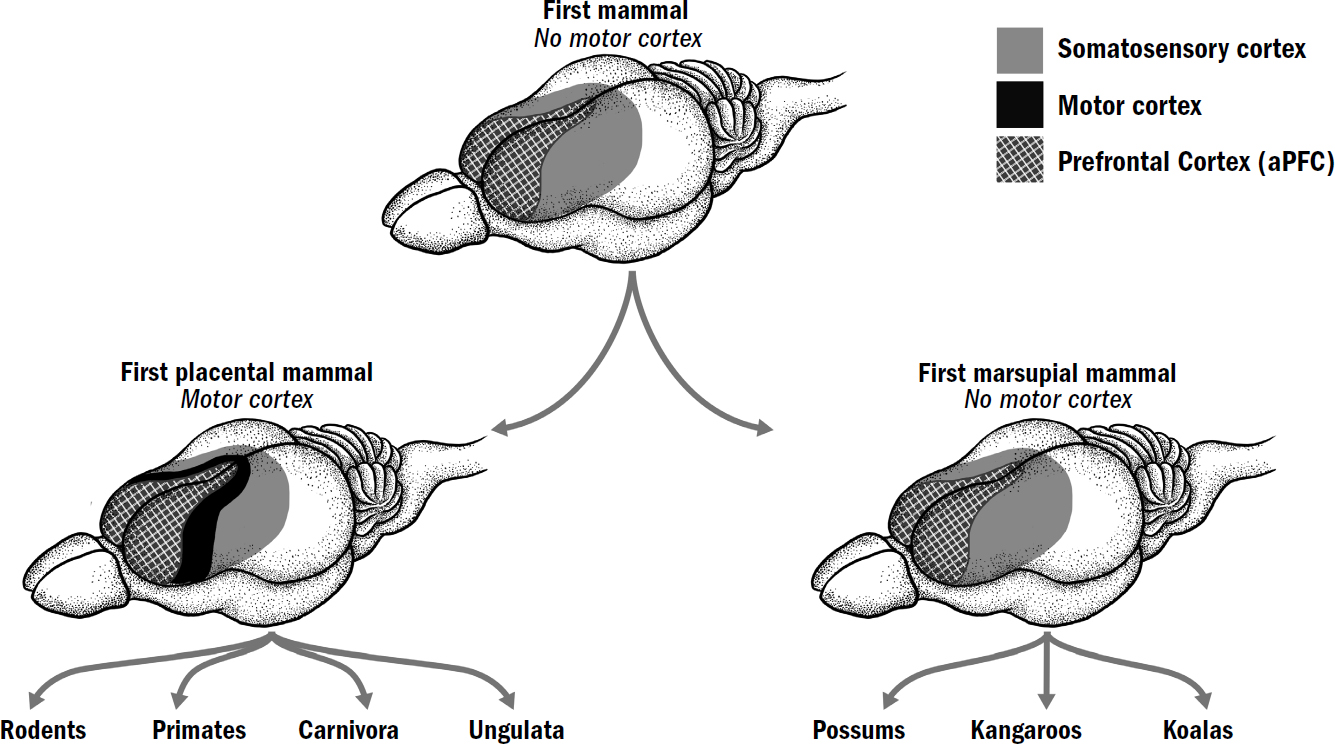
Original art by Mesa Schumacher
Predictions, Not Commands
Karl Friston, the pioneer of the theory of active inference, offers an alternative interpretation of the motor cortex. While the prevailing view has always been that the motor cortex generates motor commands, telling muscles exactly what to do, Friston flips this idea on its head: Perhaps the motor cortex doesn’t generate motor commands but rather motor predictions. Perhaps the motor cortex is in a constant state of observing the body movements that occur in the nearby somatosensory cortex (hence why there is such an elegant mirror of motor cortex and somatosensory cortex) and then tries to explain the behavior and use these explanations to predict what an animal will do next. And perhaps the wiring is merely tweaked so that motor cortex predictions flow to the spinal cord and control our movement—in other words, the motor cortex is wired to make its predictions come true.
By this account, the motor cortex operates the same way the agranular prefrontal cortex operates. The difference is that the aPFC learns to predict movements of navigational paths, whereas the motor cortex learns to predict movements of specific body parts. The aPFC will predict that an animal will turn left; the motor cortex will predict that the animal will place its left paw exactly on a platform.
This is the general idea of “embodiment”—parts of the neocortex, such as the motor cortex and somatosensory cortex, have an entire model of an animal’s body that can be simulated, manipulated, and adjusted as time unfolds. Friston’s idea explains how the neocortical microcircuit could be repurposed to produce specific body movements.
But if most mammals can move around normally with no motor cortex, then what was its original function? If the aPFC enables the planning of navigational routes, what did the motor cortex enable?
This suggests that the motor cortex was originally not the locus of motor commands but of motor planning. When an animal must perform careful movements—placing a paw on a small platform or stepping over an out-of-sight obstacle—it must mentally plan and simulate its body movements ahead of time. This explains why the motor cortex is necessary for learning new complex movements but not for executing well-learned ones. When an animal is learning a new movement, the motor cortex simulations vicariously train the basal ganglia. Once a movement is well learned, the motor cortex is no longer needed.
Original art by Rebecca Gelernter
A Hierarchy of Goals: A Balance of Simulation and Automation
How does all this work together? The frontal neocortex of early placental mammals was organized into a hierarchy. At the top of the hierarchy was the agranular prefrontal cortex, where high-level goals are constructed based on amygdala and hypothalamus activation. The aPFC might generate an intent like “drink water” or “eat food.” The aPFC then propagates these goals to a nearby frontal region (the premotor cortex), which constructs subgoals and propagates these subgoals further until they reach the motor cortex, which then constructs sub-subgoals. The intent modeled in the motor cortex are these sub-subgoals, which can be as simple as “Position my index finger here and my thumb over here.”
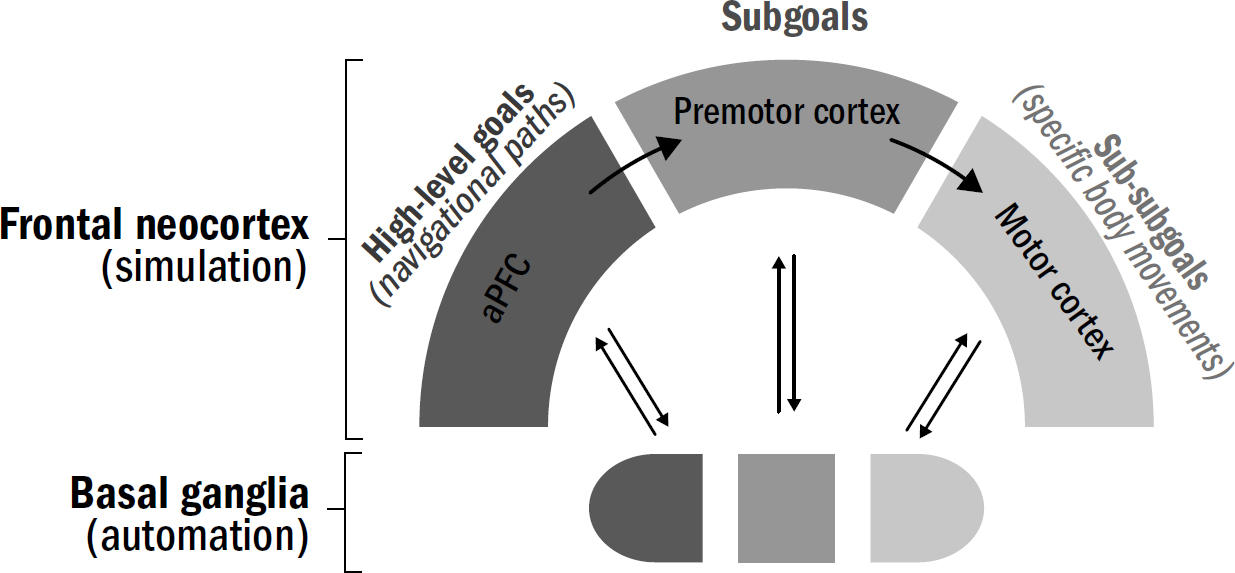
Figure 14.4: The motor hierarchy in early placental mammals
Original art by Rebecca Gelernter
This hierarchy enables more efficient processing by distributing effort across many different neocortical columns. The aPFC doesn’t have to worry about the specific movements necessary to achieve its goals; it must worry only about high-level navigational paths. Similarly, this allows the motor cortex not to have to worry about the high-level goal of the behavior and worry only about accomplishing specific low-level movement goals (picking up a cup or playing a specific chord).
The basal ganglia makes loops of connectivity with the frontal cortex, with the aPFC connecting to the front region of the basal ganglia (which then connects back to aPFC through the thalamus), and the motor cortex connecting to the back region of the basal ganglia (which then connects back to the motor cortex through a different region of the thalamus). These loops are so elegantly and particularly wired that it is hard to resist trying to reverse-engineer what they are doing.
The leading view among neuroscientists is that these are subsystems designed to manage different levels of the motor hierarchy. The front part of the basal ganglia automatically associates stimuli with high-level goals. It is what generates cravings: You come home and smell rigatoni, and suddenly you are on a mission to eat some. Drug addicts show extreme activations of this front part of the basal ganglia when they see stimuli that create drug cravings. The aPFC, however, is what makes you pause and consider if you actually want to pursue these cravings (“What about our diet?”). The back part of the basal ganglia automatically associates stimuli with low-level goals, such as specific body movements. It is what generates automatic skilled movements. The motor cortex, on the other hand, is what makes you pause and plan out your exact movements ahead of time.
HIGH-LEVEL GOALS | LOW-LEVEL GOALS | |
SIMULATION | Agranular Prefrontal Cortex Simulates navigational paths Asks “do I want rigatoni or would I rather diet?” Damage causes impairments in planning navigational routes | Motor Cortex Simulates body movements Asks “how do I configure my fingers to play this C chord that I just learned on guitar?” Damage causes impairments in learning new motor skills and executing fine motor skills |
AUTOMATION | Front part of basal ganglia Automatic pursuit of a high-level goal in response to stimulus Produces habitual cravings Damage | Back part of basal ganglia Automatic execution of motor skill in response to stimulus Produces habitual motor responses Damage causes impairments in executing learned skills, and impairs motor habit formation |
Any level of goal, whether high-level or low-level goals, has both a self model in the frontal neocortex and a model-free system in the basal ganglia. The neocortex offers a slower but more flexible system for training, and the basal ganglia offers a faster but less flexible version for well-trained paths and movements.
There is also plenty of evidence for the idea that the frontal neocortex is the locus of simulation, while the basal ganglia is the locus of automation. Damaging an animal’s motor cortex impairs movement planning and learning new movements but not the execution of well-trained movements (because the back part of the basal ganglia already learned them). Similarly, damaging an animal’s aPFC impairs path planning and learning new paths but not the execution of well-trained paths.
An intact and well-functioning motor hierarchy would have made the behavior of early placental mammals impressively flexible; animals could set high-level goals in the aPFC while lower-level areas of the motor hierarchy could flexibly respond to whatever obstacles present themselves. A mammal pursuing faraway water could continuously update its subgoals as events unfolded—the premotor cortex could respond to surprising obstacles by selected new movement sequences, and the motor cortex could adjust even the subtlest of specific movements of limbs, all in the name of a common goal.
The secret to dishwashing robots lives somewhere in the motor cortex and the broader motor system of mammals. Just as we do not yet understand how the neocortical microcircuit renders an accurate simulation of sensory input, we also do not yet understand how the motor cortex simulates and plans fine body movements with such flexibility and accuracy and how it continuously learns as it goes.
But if we use the past few decades as our guide, roboticists and AI researchers will likely figure this out, perhaps in the near future. Indeed, robotics are improving at a rapid pace. Twenty years ago, we could barely get a four-legged robot to balance itself upright, and now we have humanoid robots that can do flips in the air.
If we successfully build robots with motor systems similar to those of mammals, they will come along with many desirable properties. These robots will automatically learn new complex skills on their own. They will adjust their movements in real time to account for perturbations and changes in the world. We will give them high-level goals, and they will be able to figure out all the subgoals necessary to achieve it. When they try to learn some new task, they will be slow and careful as they simulate each body movement before they act, but as they get better, the behavior will become more automatic. Over the course of their lifetimes, the speed with which they learn new skills will increase as they reapply previously learned low-level skills to newly experienced higher-level goals. And if their brains work at all like mammal brains, they will not require massive supercomputers to accomplish these tasks. Indeed, the entire human brain operates on about the same amount of energy as a lightbulb.
Or maybe not. Perhaps roboticists will get all this to work in a very nonmammalian way—perhaps roboticists will figure it all out without reverse-engineering human brains. But just as bird wings were an existence proof for the possibility of flight—a goal for humans to strive for—the motor skills of mammals are our existence proof for the type of motor skills we hope to build into machines one day, and the motor cortex and the surrounding motor hierarchy are nature’s clues about how to make it all work.
Summary of Breakthrough #3: Simulating
The primary new brain structure that emerged in early mammals was the neocortex. With the neocortex came the gift of simulation—the third breakthrough in our evolutionary story. To summarize how this occurred and how it was used:
- Sensory neocortex evolved, which created a simulation of the external world (a world model).
- The agranular prefrontal cortex (aPFC) evolved, which was the first region of the frontal neocortex. The aPFC created a simulation of an animal’s own movements and internal states (a self model) and constructed “intent” to explain one’s own behavior.
- The aPFC and sensory neocortex worked together to enable early mammals to pause and simulate aspects of the world that were not currently being experienced—in other words, model-based reinforcement learning.
- The aPFC somehow solved the search problem by intelligently selecting paths to simulate and determining when to simulate them.
- These simulations enabled early mammals to engage in vicarious trial and error—to simulate future actions and decide which path to take based on the imagined outcomes.
- These simulations enabled early mammals to engage in counterfactual learning, thereby offering a more advanced solution to the credit assignment problem—enabling mammals to assign credit based on causal relationships.
- These simulations enabled early mammals to engage in episodic memory, which allowed mammals to recall past events and actions, and use these recollections to adjust their behavior.
- In later mammals, the motor cortex evolved, enabling mammals to plan and simulate specific body movements.
Our mammalian ancestors from a hundred million years ago weaponized the imaginarium to survive. They engaged in vicarious trial and error, counterfactual learning, and episodic memory to outplan dinosaurs. Our ancestral mammal, like a modern cat, could look at a set of branches and plan where it wanted to place its paws. Together, these ancient mammals behaved more flexibly, learned faster, and performed more clever motor skills than their vertebrate ancestors.
Most vertebrates at the time, as with modern lizards and fish, could still move quickly, remember patterns, track the passage of time, and intelligently learn through model-free reinforcement learning, but their movements were not planned.
And so, thinking itself was born not within the clay creatures of Prometheus’s divine workshop, but instead in the small underground tunnels and knotted trees of a Jurassic Earth, birthed from the crucible of a hundred million years of dinosaur predation and our ancestor’s desperate attempt to avoid fading into extinction. That is the real story of how our neocortex and our inner simulation of the world came into being. And as we will soon see, it was from this hard-won superpower that the next breakthrough would eventually emerge.
This next breakthrough has been, in some ways, the hardest breakthrough to reverse engineer in modern AI systems; indeed, this next breakthrough is a feat we don’t typically associate with “intelligence,” but is, in fact, one of our brain’s most impressive feats.
Breakthrough #4
Mentalizing and the First Primates
Your brain 15 million years ago
Original art by Rebecca Gelernter
15
The Arms Race for Political Savvy
IT HAPPENED ON some unremarkable day around sixty-six million years ago, a day that began no different than any other. The sun rose over the jungles of today’s Africa, awakening slumbering dinosaurs and driving our nocturnal squirrel-like ancestors into their daytime hiding spots. Along the muddy seashores, rain pattered into shallow ponds filled with ancient amphibians. Tides receded, drawing the many fish and other ancient critters deep in the oceans. The skies filled with pterosaurs and ancient birds. Arthropods and other invertebrates tunneled within the soils and trees. The ecology of Earth had found a beautiful equilibrium, with dinosaurs comfortably at the top of the food chain for well over a hundred and fifty million years, fish ruling the sea for even longer, and mammals and other animals finding their respective tiny but livable niches. Nothing hinted that this day would be any different than any other, but it was indeed this day when everything changed—this was the day the world almost ended.
The specific life stories of any of the animals who experienced this day are, of course, lost to us. But we can speculate. One of our mammalian squirrel-like ancestors was perhaps on her way out of her burrow to begin a night of scavenging insects. As the sun was just beginning to set, the sky must have turned that purple hue it did every other evening. But then blackness emerged from the horizon. A dark cloud, thicker than any storm she had ever seen, spread rapidly over the sky. Perhaps she looked up at this novel sight with puzzlement; perhaps she ignored it completely. Either way, despite all her new neocortical smarts, she would have had no way to understand what was happening.
This was no storm on the horizon—this was space dust. Just a few minutes prior, on the other side of the planet, an asteroid a few miles wide had slammed into the Earth. It had sent up gargantuan chunks of earth debris that was rapidly filling the skies with dark soot—a blackness that would block out the sun for over one hundred years, killing over 70 percent
Many of the other extinction events in the history of Earth seem to have been self-imposed—the Great Oxygenation Event was caused by cyanobacteria, and the Late Devonian Extinction was possibly caused by overproliferation of plants on land. But this one was not a fault of life but a fluke of an ambivalent universe.
Eventually, after over a hundred years, the blackened clouds began to fade. As the sun reemerged, plants began recovering lost ground and refilling the parched dead land. But this was a new world. Almost every dinosaur species was extinct except for one: the birds. Although our squirrel-like ancestor could not have known it and had not lived to see it, her offspring would inherit this new Earth. As the Earth healed, these small mammals found themselves in a completely new ecological playground. Without their dinosaur predators, they were free to explore new ecological niches, to diversify into new shapes and sizes, to conquer new territories, and to find new footing within the food chain.
The era that followed has been called the Era of Mammals. Descendants of these early mammals would eventually evolve into modern-day horses, elephants, tigers, and mice. Some would even reenter the sea and become today’s whales, dolphins, and seals. Some would take to the sky and become today’s bats.
Our direct ancestors were the ones who found refuge in the tall trees of Africa. These were some of the first primates. They shifted from being night-living (nocturnal) to being day-living (diurnal). As they became larger, they developed opposable thumbs to grasp branches and hold their heavier bodies. To support their bigger size, they shifted from an insect-based diet to a fruit-based diet. They lived in groups, and as they grew, they became relatively free from predation and food competition. And most notably, their brains exploded to well over a hundred times their original size.
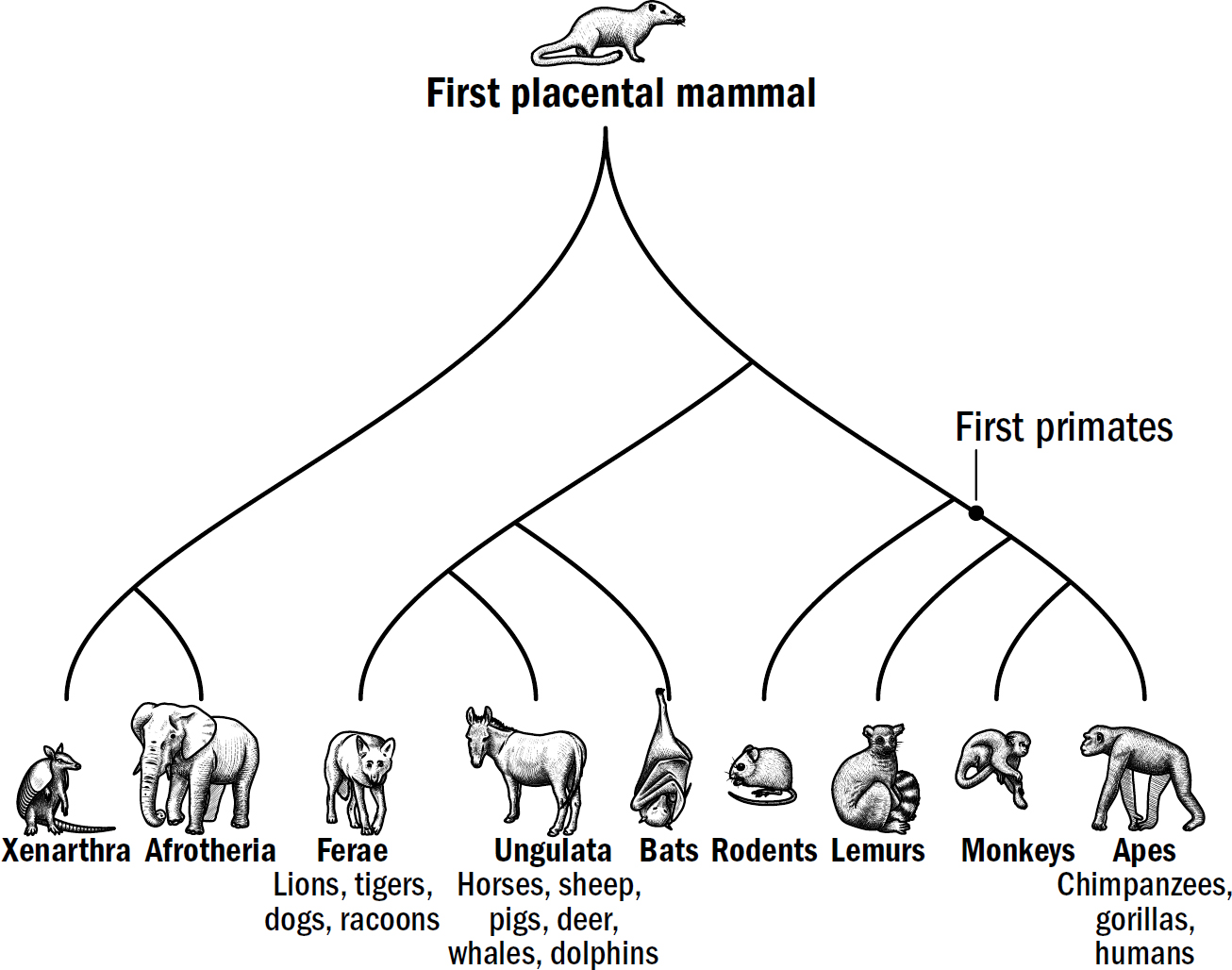
Figure 15.1: Tree of mammals
Original art by Rebecca Gelernter
Many lineages of mammals went on to have brains not much larger (proportionally) than those of early mammals, it was only in certain lineages of mammals, such as those of elephants, dolphins, and primates, where brains dramatically expanded. Because this book is about the human story, we will focus on the journey by which the primate brain became big. Indeed, why primates have such big brains—and specifically such large neocortices—is a question that has perplexed scientists since the days of Darwin. What was it about the lifestyle of early primates that necessitated such a big brain?
The Social-Brain Hypothesis
In the 1980s and 1990s, numerous primatologists and evolutionary psychologists, including Nicholas Humphrey, Frans de Waal, and Robin Dunbar, began speculating that the growth of the primate brain had nothing to do with the ecological demands of being a monkey in the African jungles ten to thirty million years ago and was instead a consequence of the unique social demands. They argued that these primates had stable mini-societies: Groups of individuals that stuck together for long periods. Scientists hypothesized that to maintain these uniquely large social groups, these individuals needed unique cognitive abilities. This created pressure, they argued, for bigger brains.
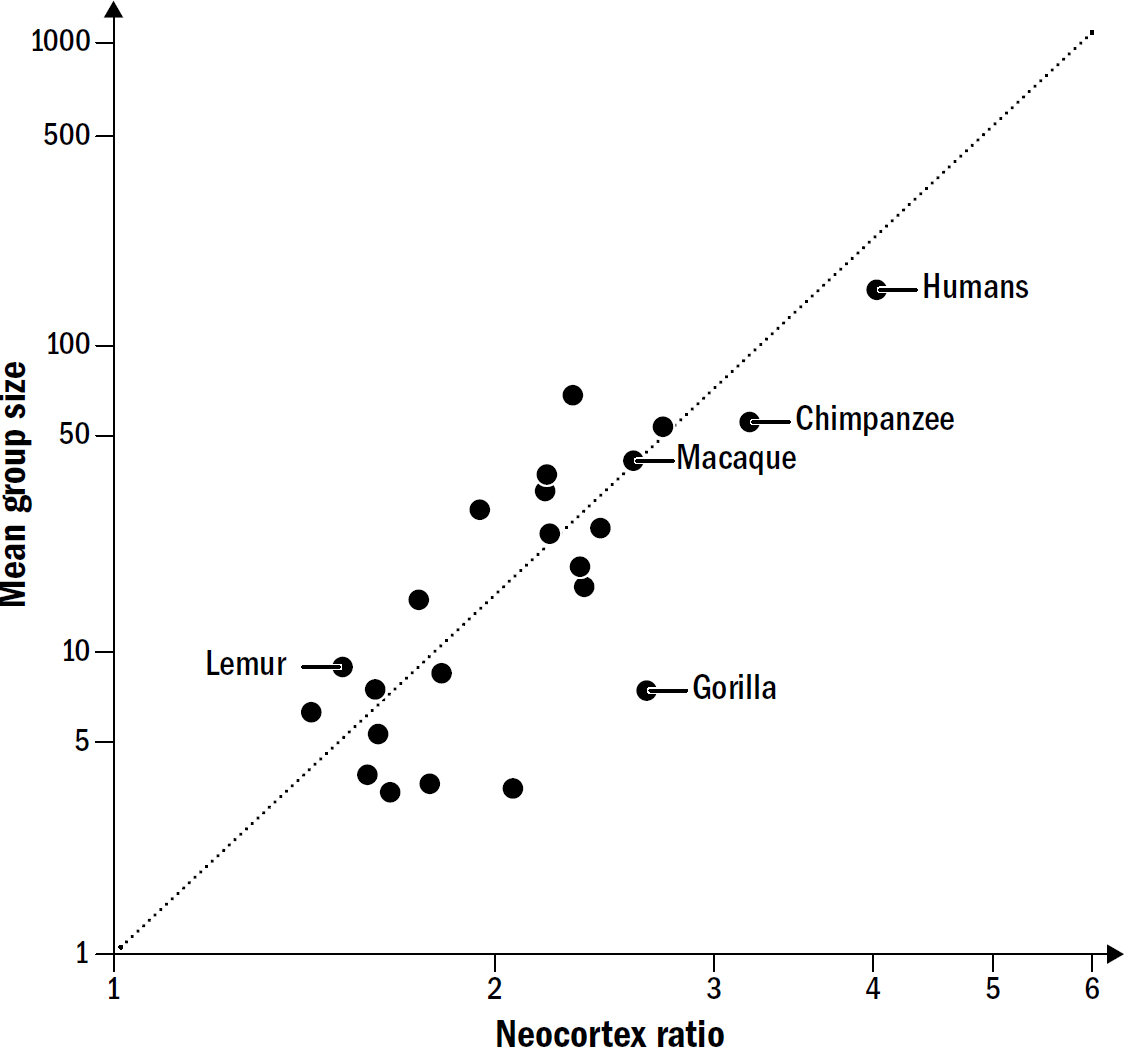
Original art by Rebecca Gelernter
The Evolutionary Tension Between the Collective and the Individual
Early mammals were likely more social than the amniotes (their lizard-like ancestors) that came before. These early mammals uniquely gave birth to helpless children. This dynamic would have been tenable only if mothers built a strong bond to help, nurture, and physically protect their children. Further, mammals engage in play much more than other vertebrates. Even the offspring of simple mammals like rats play with each other, play mounting and play fighting. These early acts of play might have served the purpose of refining and training the motor cortex of young mammals so that in higher-stake situations they wouldn’t be learning from scratch. In these early mammals, this collaborative period between mother and child was relatively short-lived. After a period of childhood development, the bond tends to fade, and the children and mothers go their separate ways. This is how it is for many mammals that spend most of their lives on their own, like tigers and bears.
But not all animals separate in adulthood like this. In fact, the simplest, most widely used, and likely first collective behavior in animals was group living, whereby animals of the same species simply clustered together. Fish reflexively follow each other’s movements and swim near one another. Many herbivorous dinosaurs lived in herds. And, of course, this is seen across mammals as well—buffalo and antelope live in herds. The key benefit of group living is that it helps stave off predators. If even a single antelope in a herd catches a glimpse of a nearby lion and begins running away, it cues all the others in the herd to follow. While a lone antelope is easy prey, a herd of them can be dangerous to even a lion.
However, group living is not a freely gained survival benefit—it comes at a high cost. In the presence of food constraints or limited numbers of eligible mates, a herd of animals creates dangerous competition. If this competition leads to infighting and violence, the group ends up wasting precious energy competing and fighting each other. In such a circumstance, the same number of animals would have been better off living separately.
Thus, animals who fell into the strategy of group living evolved tools to resolve disputes while minimizing the energetic cost of such disputes. This led to the development of mechanisms to signal strength and submission without having to actually engage in a physical altercation. Deer and antelope lock horns to compete for food and mates, a much cheaper form of competition than fighting. Bears, monkeys, and dogs bare their teeth and growl to show aggression.
SOLITARY | PAIR-BONDING | HAREMS | MULTI-MALE GROUPS |
Independent | One male, one female | One male, many females | Many males, many females |
Live mostly independently in adulthood | One male and one female live and raise children together. | A single dominant male living with a group of females who have their own hierarchy. | Separate male and female hierarchy |
Tigers Jaguar Moose | Red foxes Prairie voles Giant otters Pygmy marmoset | Mongolian camels Fur seals Gorillas | Lions Hippopotamuses Lemurs Chimpanzees Baboons Macaque monkeys |
Multi-male groups also work via hierarchical rigidity: There is a strict hierarchy of both males and females. Low-ranking males are allowed in the group, but they do little mating and get the last pick of food; the high-ranking males eat food first and do most, if not all, of the mating.
How is the hierarchy decided in these social groups? It’s simple—the strongest, biggest, and toughest become dominant. The locking of horns and baring of teeth are all designed to demonstrate who would win in a fight while avoiding the fight itself.
Machiavellian Apes
In the 1970s, the primatologist Emil Menzel was running experiments with a group of chimpanzees
Menzel would hide some food in a random location within this one-acre area, perhaps under a rock or within a bush, and then reveal its location to one of the chimpanzees. He would then place food back in these locations on a recurring basis. Chimpanzees, like rats, were eminently able to remember these exact locations, learning to recheck these specific spots to forage Menzel’s hidden food. But Menzel began spotting behavior that was quite unlike that of a rat, behavior that he had never intended to investigate and had never expected to find. Indeed, while merely investigating spatial memory, Menzel unearthed behavior that was eerily Machiavellian.
When Menzel first revealed the location of hidden food to one of the subordinate chimpanzees, named Belle, she happily alerted the rest of the group and shared the food. But when the dominate male, Rock, came over to indulge in the treat, he took all the food for himself. After Rock did this a few times, Belle stopped sharing, and began engaging in ever more sophisticated strategies to withhold information about the hidden locations of food from Rock.
At first, Belle simply sat on top of the secret location of the food to hide it from Rock, and only when he was far away would she uncover and openly eat the food. But when Rock realized she was hiding the food underneath her, he began pushing her to get at the food. In response to this, Belle came up with a new strategy—once she was shown a new location for the hidden food, she didn’t go to it immediately. She would wait for Rock to look away, and then she would run to the food. In response to this new strategy, Rock began trying to trick Belle: He would look away and act uninterested, and once Belle went for the food, he would turn around and run toward it. Belle even began trying to lead Rock in the wrong directions, a deception Rock eventually realized and thus, in response, began to search for food in the opposite direction that Belle would try to lead him.
This act of inferring someone’s intent and knowledge is called “theory of mind”—so named because it requires us to have a theory about the minds of others. It is a cognitive feat that evidence suggests emerged in early primates. And as we will see, theory of mind might explain why primates have such big brains and why their brain size correlates with group size.
Primate Politics
What makes these monkey societies unique is not the presence of a social hierarchy (many animal groups have social hierarchies), but how the hierarchy is constructed. If you examined the social hierarchy of different monkey groups, you would notice that it often isn’t the strongest, biggest, or most aggressive monkey who sits at the top. Unlike most other social animals, for primates, it is not only physical power that determines one’s social ranking but also political power.
The Arms Race for Political Savvy
Somehow the evolutionary trajectory of early primates led to the development of the incredibly broad suite of complex social behaviors that are seen across modern species of primates. And within these behaviors we see hints of the behavioral foundation of how humans tend to interact with each other. Why primates evolved these instincts is not exactly clear, but it may have had to do with the unique niche early primates found themselves in in the aftermath of the Permian-Triassic extinction event.
Early primates seemed to have had a unique diet of foraging fruit directly in treetops—they were frugivores. They plucked fruit from trees right after it ripened but before it fell to the forest floor. This allowed primates to have easy access to food without much competition from other species. This unique ecological niche may have offered early primates two gifts that opened the door to their uniquely large brains and complex social groups. First, easy access to fruit gave early primates an abundance of calories, providing the evolutionary option to spend energy on bigger brains. And second, and perhaps more important, it gave early primates an abundance of time.
It isn’t clear how political savviness would even be possible if a species did not have at least a basic and primitive version of theory of mind—only through this ability can individuals infer what others want and thereby figure out whom to cozy up to and how. Only through theory of mind can individual primates know not to mess with a low-ranking individual with high-ranking friends; this requires understanding the intent of the high-ranking individuals and what they will do in future situations. Only through this ability of theory of mind can you figure out who is likely to become powerful in the future, whom you need to make friends with, and whom you can deceive.
So this may be why primates began growing such big brains, why their brain size is correlated with social group size, and why primates evolved the ability to reason about the minds of others. The question is, of course, how do primate brains do this?
16
How to Model Other Minds
OUR MAMMALIAN ANCESTOR from seventy million years ago had a brain that weighed less than half a gram. By the time our ape ancestors arrived ten million years ago, it had expanded to
Clearly some structures in brains will scale naturally with body size without any meaningful change to their function. For example, a bigger body means more touch and pain nerves, which means more neocortical space for processing these sensory signals. The surface area of the somatosensory cortex of the early apes was obviously much bigger than that of early mammals even though it performed the same function. Same for bigger eyes and muscles and anything else that requires incoming or outgoing nerves.
Further, more neurons can be added to a structure to improve its performance without fundamentally changing its function. For example, if the basal ganglia were one hundred times larger, it might enable associations between many more actions and rewards while still fundamentally performing the same function: implementing a temporal difference learning algorithm. Similarly, the visual cortex of primates is massively larger than that of rodents, even accounting for brain scaling. Unsurprisingly, primates are better than rodents at many aspects of visual processing. But the visual area of the neocortex does not perform some unique function in primates; primates simply dedicated more space, proportionally, to the same function and got better performance.
And then there are the fuzzy areas—those structures that are very similar but slightly modified, teetering on the edge of new and old. An example of this is new hierarchical layers of sensory processing in the neocortex. Primates have many hierarchical regions of the visual neocortex with processing hopping from one region to the next. These areas still process visual input, but the addition of new hierarchical layers makes them qualitatively different. Some areas respond to simple shapes; other areas respond to faces.
But there are also, of course, truly new brain regions—structures with completely unique connectivity that perform novel functions.
So might it be the case that the surprising smarts of primates, with all their theory of mind, politicking, and trickery, was a consequence of nothing more than brain scaling?
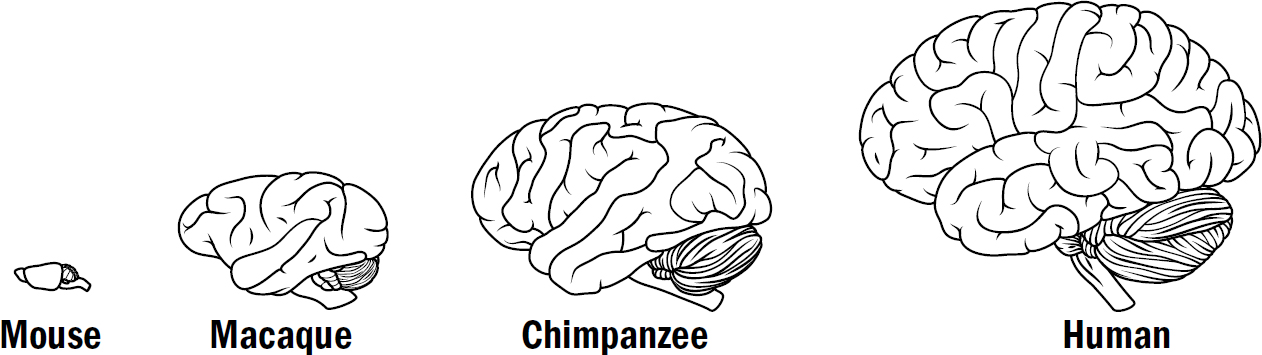
Figure 16.1
Original art by Rebecca Gelernter
The New Neocortical Regions of Early Primates
What makes these areas “new”? It isn’t their microcircuitry; all these areas are still neocortex and have the same general columnar microcircuitry as other areas of neocortex across mammals. It is their input and output connectivity that renders them new; it is what these areas construct a generative model of that unlocked fundamentally new cognitive abilities.
In contrast to the alarming symptoms of aPFC damage, damage to the surrounding granular prefrontal cortex often results in minimal symptoms. In fact, the impairment from damage to these areas is so minimal that many neuroscientists in the 1940s wondered if these areas lacked any
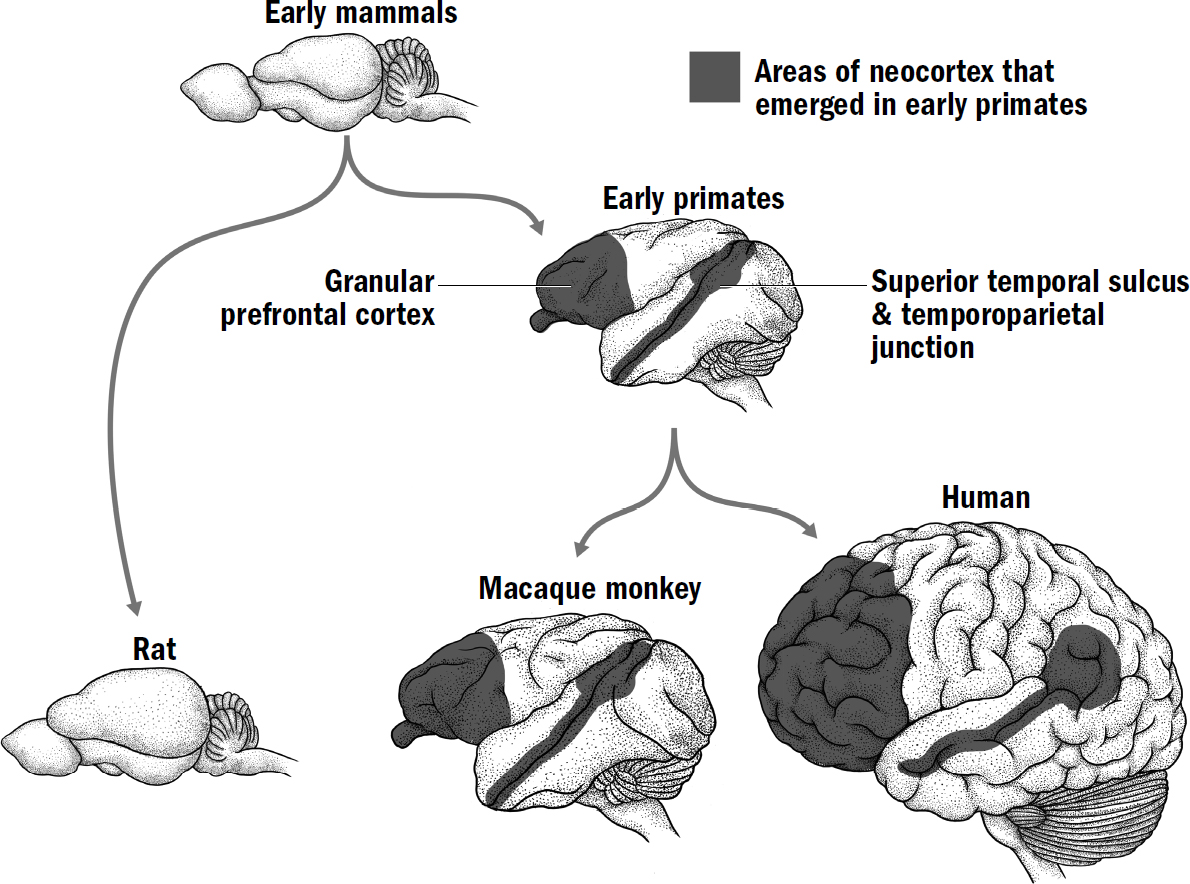
Figure 16.2: Shared neocortical regions across mammals and new regions of primates
Original art by Mesa Schumacher
Modeling Your Own Mind
With this clue the granular prefrontal cortex is uniquely activated by self-reference, might we have missed some subtle but crucial impairments of granular prefrontal damage?
In 2015, scientists did the following study. They gave participants a neutral cue word (e.g., bird or restaurant) and asked them to tell the experimenter different narratives of themselves associated with that word. Some of these participants were healthy, some had damage to areas of the granular prefrontal cortex, and some had damage to the hippocampus.
This suggests that the granular prefrontal cortex plays a key role in your ability to project yourself—your intentions, feelings, thoughts, personality, and knowledge—into your rendered simulations, whether they are about the past or some imagined future. The simulations run in rat brains, which has no gPFC and only an aPFC and hippocampus, show evidence of rendering an external world, but there is nothing to suggest that they truly project any meaningful model of themselves into these simulations.
One interpretation of this is that these new primate areas are constructing a generative model of the older mammalian aPFC and sensory cortex itself. Just as aPFC constructs explanations of amygdala and hippocampus activity (invents “intent”), perhaps the gPFC constructs explanations of the aPFC’s model of intent—possibly inventing what one might call a mind. Perhaps the gPFC and PSC construct a model of one’s own inner simulation to explain one’s intentions in the aPFC given knowledge in the sensory neocortex.
Let’s use a thought experiment to build some intuition about what this means. Suppose you put our ancestral primate in a maze. When it reached a choice point, it turned left. Suppose you could ask its different brain areas why the animal turned left. You would get very different answers at each level of abstraction. Reflexes would say, Because I have an evolutionarily hard-coded rule to turn toward the smell coming from the left. Vertebrate structures would say, Because going left maximizes predicted future reward. Mammalian structures would say, Because left leads to food. But primate structures would say, Because I’m hungry, eating feels good when I am hungry, and to the best of my knowledge, going left leads to food. In other words, the gPFC constructs explanations of the simulation itself, of what the animal wants and knows and thinks. Psychologists and philosophers call this metacognition: the ability to think about thinking.
What mammals find in their inner simulations of the external world is, in some sense, the same thing as their knowledge about the external world. When a mammal simulates going down a path and its sensory neocortex renders a simulation that contains water at the end of the path, this is the same thing as “knowing” that water exists at the end of the path. While the older mammalian areas of the sensory neocortex render the simulation of the external world (containing knowledge), the new primate areas of neocortex (what I have been calling the primate sensory cortex) seems to create a model of this knowledge itself (areas of PSC get input from various areas of sensory neocortex). These new primate areas try to explain why the sensory neocortex believes food is over there, why an animal’s inner simulation of the external world is the way it is. An answer might be: Because last time I went over there I saw water, and hence when I simulate going back there, I see water in my imagination. Put slightly differently: Because I last saw water there, I now know that water is over there even though before I did not.
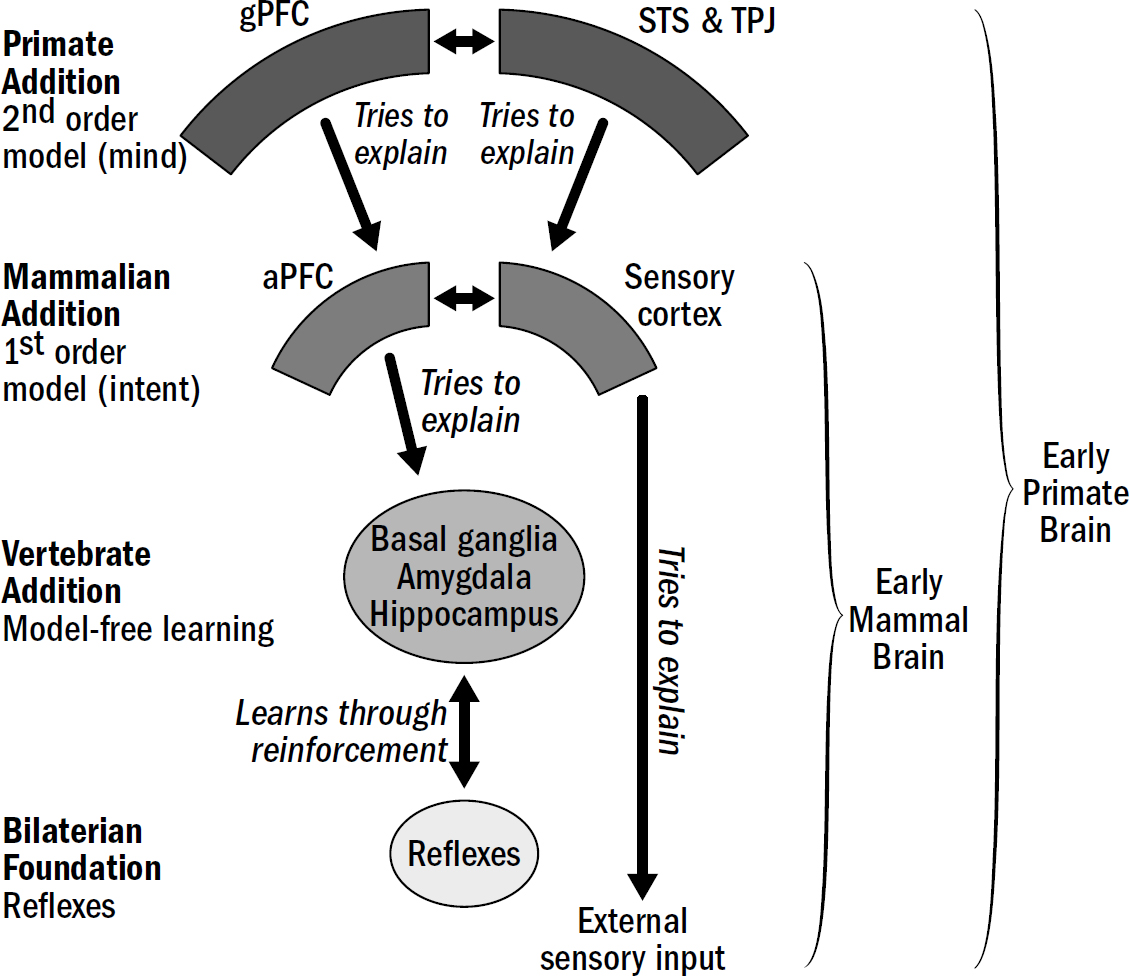
Figure 16.3
Original art by Rebecca Gelernter
These systems are all bootstrapped on one another. Reflexes drive valence responses without any learning required, making choices based on evolutionarily hard-coded rules. The vertebrate basal ganglia and amygdala can then learn new behaviors based on what has historically been reinforced by these reflexes, making choices based on maximizing reward. The mammalian aPFC can then learn a generative model of this model-free behavior and construct explanations, making choices based on imagined goals (e.g., drinking water). This could be considered a first-order model. The primate gPFC can then learn a more abstract generative model (a second-order model) of this aPFC-driven behavior and construct explanations of intent itself, making choices based on mind states and knowledge (I’m thirsty; drinking water when thirsty feels good, and when I simulate going down this way, I find water in my simulation, hence I want to go in this direction).
The mammalian first-order model has a clear evolutionary benefit: It enables the animal to vicariously play out choices before acting. But what is the evolutionary benefit of going through the trouble of developing a second-order model? Why model your own intent and knowledge?
Modeling Other Minds
Consider the comic-strip task devised by Eric Brunet-Gouet in 2000. Human participants were shown several comic strips, each containing three frames, and asked to guess which fourth-frame ending was most likely. There were two types of comic strips—one required inferring the intent of characters in order to correctly guess the ending, and the other required only understanding physical causal relationships.
In addition to inferring other people’s intent, these primate areas also get activated by tasks that require inferring other people’s knowledge. A famous test of this is the Sally-Ann test: Participants are shown a series of events occurring between two individuals, Sally and Ann. Sally puts a marble in a basket; Sally leaves; when Sally isn’t looking, Ann moves the marble from the basket to a nearby box; Sally comes back. The participant is then asked: If Sally wants to play with her marble, where will she look for it?
Images from Brunet et al., 2000; Völlm et al., 2006; and personal correspondence with Dr. Eric Brunet-Gouet. Used with permission of Dr. Brunet-Gouet (personal correspondence).
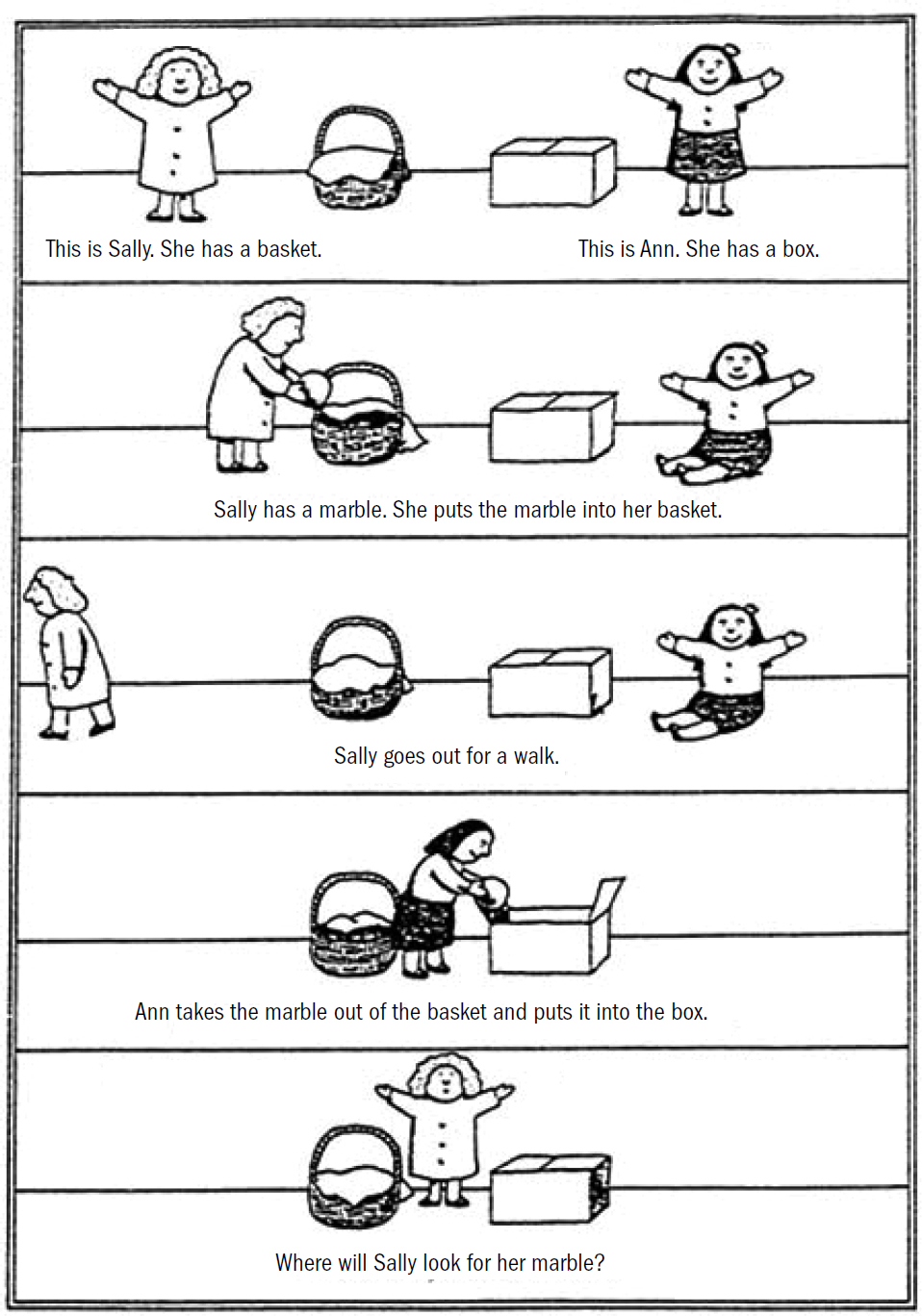
Photo from Frith, 2003. Reused with permission.
Modeling Your Mind to Model Other Minds
At the top of the social hierarchy of a troop of ancestral primates was more access to food and mates, and at the bottom was last pick of food and no access to mates. Theory of mind enabled each primate to climb this social ladder; it enabled them to manage their reputation and hide their transgressions; it enabled them to forge allyships, cozy up to rising stars, and kiss the ring of powerful families; it enabled them to build coalitions and stage rebellions; it enabled them to ameliorate brewing disputes and repair relationships after a tiff. Unlike the intellectual abilities that had emerged in the breakthroughs prior, theory of mind was not born from the need to survive the dangers of hungry predators or inaccessible prey, but instead from the subtler and far more cutting dangers of politics.
Politics was the origin story of Breakthrough #4, but it is far from the entire story. As we will see in the next two chapters, theory of mind in early primates was repurposed for two other new abilities.
17
Monkey Hammers and Self-Driving Cars
JANE GOODALL COULD not believe her eyes.
It was November 1960. For months, she had been following a local tribe of chimpanzees in Gombe, Tanzania. The chimps had only recently begun accepting her presence, allowing her to stay close enough to observe them in their natural habitat. In the years before, Goodall had befriended a Kenyan paleontologist by the name of Louis Leakey; he eventually offered to send her to study the social lives of chimpanzees in their natural habitat. But the first discovery Goodall would make was not about their social lives.
As Goodall sat quietly some distance away, she noticed two chimpanzees that she had named David Greybeard and Goliath grabbing thin branches, stripping the leaves off them, and sticking them into a termite mound. When they pulled them out, they were covered in tasty termites, which they gobbled up. They were fishing. They were using tools.
It was long assumed that tool use was uniquely human, but tool use has now been found across many primates. Monkeys and apes not only use sticks to fish termites; they also use rocks
If the driver of brain evolution in early primates was a politicking arms race, why would primates be uniquely good tool users? If the new brain regions of primates were “designed” to enable theory of mind, then from where do the unique tool-using skills of primates emerge?
Monkey Mirrors
After trying to replicate this phantom sandwich-watching activation, Rizzolati’s team quickly realized that they had, in fact, discovered something more general: when their monkey observed a human perform a motor skill—whether picking up a peanut with two fingers, grasping an apple with their full hand, or grasping a snack with their mouth—the monkey’s own motor neurons for performing that same skill would often activate. In other words, the neurons in the premotor and motor areas of a monkey’s neocortex—those that control a monkey’s own movements—not only activated when they performed those specific fine motor skills, but also when they merely watched others perform them. Rizzolatti called these “mirror neurons.”
Here is some evidence that mirror neurons are just imagined movements. Monkeys don’t need to directly observe the movements for their mirror neurons to activate; they can merely be given sufficient information for them to infer what movements are being performed. The motor neurons that light up right before a monkey does a behavior (such as picking up a peanut with the intention of breaking it open) also activate if a monkey simply hears the peanut break
One reason it is useful to simulate other people’s movements is that doing this helps us understand their intentions. By imagining yourself doing what others are doing, you can begin to understand why they are doing what they are doing: you can imagine yourself tying strings on a shoe or buttoning a shirt and then ask yourself “why would I do something like this?” and thereby begin to understand the underlying intentions behind other people’s movements. The best evidence for this is found in the bizarre fact that people with impairments in performing specific movements, also show impairments in understanding the intentions of those very same movements in others. The subregions of premotor cortex required for controlling a given set of motor skills are the same subregions required for understanding the intentions of others performing those same motor skills.
For example, in patients with brain damage to motor areas of neocortex, there is a significant correlation between impairments to action production (the ability to correctly mime using tools such as toothbrushes, combs, forks, or erasers) and action recognition (the ability to correctly select a video of a mimed action that matches an action
Suppose you put a novice guitar player in an fMRI machine and ask them to learn a guitar chord by watching a video of an expert guitarist playing that chord. And suppose you compare their brain activation under two conditions, the first being when they observe a chord they don’t know yet and the second when they observe a chord they already know how to play. The result: when they observe a chord they do not yet know, their premotor cortex
Transmissibility Beats Ingenuity
Think about all the clever motor skills related to using tools: typing, driving, brushing your teeth, tying a tie, or riding a bicycle. How many of these skills did you figure out on your own? I’m going to bet that practically all these skills were acquired by observing others, not by your own independent ingenuity. Tool use in nonhuman primates originates the same way.
The ability to use tools is less about ingenuity and more about transmissibility. Ingenuity must occur only once if transmissibility occurs frequently; if at least one member of a group figures out how to manufacture and use a termite-catching stick, the entire group can acquire this skill and continuously pass it down throughout generations.
SELECTING KNOWN SKILLS THROUGH OBSERVATION | ACQUIRING NOVEL SKILLS THROUGH OBSERVATION |
Many mammals Octopuses Fish Reptiles | Primates Some birds |
But there is a difference between observational learning in primates relative to most other mammals. If a parent mongoose tends to break open eggs with its mouth, then so does its offspring; if a parent mongoose tends to break open eggs by throwing, then so does its offspring. But these children mongooses aren’t acquiring a novel skill by watching; they are merely changing which technique they tend to use—all children mongooses exhibit both biting and throwing tricks for opening eggs. Kittens learn to pee in litter boxes only if exposed to their mothers doing it, but all kittens know how to pee. Fish don’t learn how to swim by watching; they merely change their paths by watching. In all these cases, animals aren’t using observational learning to acquire novel skills; they are merely selecting a known behavior based on seeing another do the same thing.
Selecting a known behavior through observation can be accomplished with simple reflexes: A tortoise may have a reflex to look in the direction that other tortoises are looking; a fish may have a reflex to follow other fish. A mouse can simulate itself pushing a lever (something it already knows how to do) when it observes another mouse pushing a lever, at which point this mouse will realize that it will get water if it does this. But acquiring an entirely novel motor skill by observation may have required, or at least hugely benefited from, entirely new machinery.
Why Primates Use Hammers but Rats Don’t
Acquiring novel skills through observation required theory of mind, while selecting known skills through observation did not. There are three reasons why this was the case. The first reason why theory of mind was necessary for acquiring novel skills by observation is that it may have enabled our ancestors to actively teach. For skills to be transmitted through a population, you don’t need teachers—dutiful observation by novices will do. But active teaching can substantially improve the transmission of skills. Think about how much harder it would have been to learn to tie your shoes if you didn’t have a teacher who slowed down and walked you through each step, and instead you had to decipher the steps on your own by watching people rapidly tie their shoes with no regard for your learning.
The second reason why theory of mind was necessary for learning novel motor skills through observation is that it enabled learners to stay focused on learning over long periods. A rat can see another rat push a lever and a few moments later push the lever itself. But a chimpanzee child will watch its mother use anvils to break open nuts and practice this technique for years without any success before it begins to master the skill. Chimp children continually attempt to learn without any near-term reward.
It is possible chimp children do this simply because they find imitation rewarding on its own, but another possibility is that theory of mind enables novices to identify the intent of a complex skill, which makes them highly motivated to keep trying to adopt it. Theory of mind enables a chimp child to realize that the reason it is not getting food with its stick while its mother is getting food is that its mother has a skill it does not yet have. This enables a continuous motivation to acquire the skill, even if it takes a long time to master. When a rat imitates behaviors, on the other hand, it will quickly give up if its actions don’t lead to a near term reward.
The third and final reason why theory of mind was necessary for learning novel motor skills through observation was that it enabled novices to differentiate between the intentional and unintentional movements of experts. Observational learning is more effective if one is aware of what another is trying to accomplish with each movement. If you watched your mother tie her own shoes and you had no idea what aspects of her movements were intentional versus accidental, it would be quite hard to decipher which movements to follow. If you realized that her intention was to get her shoes tied, that when she slipped it was an accident, and that both the way she is seated and the angle of her head are irrelevant aspects of the skill, it would be much easier for you to learn the skill through observation.
Understanding the intentions of movements is essential for observational learning to work; it enables us to filter out extraneous movements and extract the essence of a skill.
Robot Imitation
In 1990, a graduate student at Carnegie Mellon named Dean Pomerleau and his adviser Chuck Thorpe built an AI system to autonomously drive a car. They called it ALVINN (Autonomous Land Vehicle in a Neural Network). ALVINN was fed video footage from around a car and could—on its own—steer the car to stay within a lane on a real highway. There had been previous attempts at autonomous cars like this, but they were very slow, often pausing every few seconds; the original version of the autonomous car in Thorpe’s group could go only a quarter of a mile per hour due to how much thinking it had to do. ALVINN was much faster, so fast, in fact, that ALVINN successfully drove Pomerleau from Pittsburgh to the Great Lakes on a real highway with other drivers.
Why was ALVINN successful while previous attempts had failed? Unlike previous attempts to build a self-driving car, ALVINN was not taught to recognize objects or plan its future movements or understand its location in space. Instead, ALVINN outperformed these other AI systems by doing something simpler: It learned by imitating human drivers.
But then Pomerleau hit a snag; it quickly became clear that this approach to imitation learning—of copying expert behavior directly—had a critical flaw. Whenever ALVINN made even small errors, it was completely unable to recover. Small mistakes would rapidly cascade into catastrophic failures of driving, often veering entirely off the road. The problem was that ALVINN was trained only on correct driving. It had never seen a human recover from a mistake because it had never seen a mistake in the first place. Directly copying expert behaviors turned out to be a dangerously brittle approach to imitation learning.
There are numerous strategies to overcome this problem in robotics. But two in particular draw conspicuous parallels to how primates seem to make imitation learning work. The first is to emulate a teacher-student relationship. In addition to training an AI system to directly copy an expert, what if the expert also drove alongside the AI system and corrected its mistakes? One of the first attempts to do this was by Stephane Ross and his adviser Drew Bagnell at Carnegie Mellon in 2009. They taught an AI system to drive in a simulated Mario Kart environment. Instead of recording himself drive and then training a system to imitate it, Ross drove around the Mario Kart track and traded control over the car with the AI system. At first, Ross would do most of the driving, then control would be passed to the AI system for a moment, and any mistakes it made would be quickly recovered by Ross. Over time, Ross gave more control to the AI system until it was driving well on its own.
This strategy of active teaching worked fantasically. When only directly copying driving (like ALVINN was trained), Ross’s AI system was still crashing cars after a million frames of expert data. In contrast, with this new strategy of active teaching, his AI system was driving almost perfectly after only
Ng and his team didn’t want to get an AI system to simply fly a helicopter, they wanted it to perform acrobatic tasks, those that only the best human experts could perform: flipping in place without falling, rolling while moving forward, flying upside down, performing arial loops, and more.
Part of their approach was standard imitation learning. Ng and his team recorded human expert inputs to the remote control as they performed these acrobatic tricks. But instead of training the AI system to directly copy the human experts (which didn’t work), they trained the AI system to first infer the intended trajectories of the experts, inferring what it seemed like the humans were trying to do, and then the AI system learned to pursue those intended trajectories. This technique is called “inverse reinforcement learning” because these systems first try to learn the reward function they believe the skilled expert is optimizing for (i.e., their “intent”), and then these systems learn by trial and error, rewarding and punishing themselves using this inferred reward function. An inverse reinforcement learning algorithm starts from an observed behavior and produces its own reward function; whereas in standard reinforcement learning the reward function is hard-coded and not learned. Even when expert pilots flew these helicopters, they were continually recovering from small mistakes. By first trying to identify the intended trajectories and movements, Ng’s AI system was both filtering out extraneous mistakes of experts, and correcting its own mistakes. Using inverse reinforcement learning, by 2010 they successfully trained an AI system to autonomously perform arial aerobatics with a helicopter.
Theory of mind evolved in early primates for politicking. But this ability was repurposed for imitation learning. The ability to infer the intent of others enabled early primates to filter out extraneous behaviors and focus only on the relevant ones (what did the person mean to do?); it helped youngsters stay focused on learning over long stretches of time; and it may have enabled early primates to actively teach each other by inferring what a novice does and does not understand. While our ancestral mammal likely could select known skills by observing others, it was with early primates, armed with theory of mind, when the ability to acquire truly novel skills through observation emerged. This created a new degree of transmissibility: skills that were discovered by clever individuals and that would once have faded when they died, could now propagate throughout a group and be passed down endlessly through generations. This is why primates use hammers and rats do not.
18
Why Rats Can’t Go Grocery Shopping
ALTHOUGH ROBIN DUNBAR’S social-brain hypothesis has, for the past several decades, held primacy among scientists as the leading explanation of brain expansion in primates, there is an alternative explanation: what has been called the ecological-brain hypothesis.
As we have seen, early primates were not only uniquely social but also had a unique diet: they were frugivores. Fruit-based diets come with several surprising cognitive challenges. There is only a small window of time when fruit is ripe and has not yet fallen to the forest floor. In fact, for many of the fruits these primates ate, this window is
Animals who feed on non-fruit plants do not have to cope with this same challenge; leaves, nectar, seeds, grass, and wood all last for long periods and are not localized in sparse patches. Even carnivores don’t have as cognitively challenging a task—prey must be hunted and outsmarted, but there are rarely only short time windows in which hunting is possible.
Part of what makes this frugivore strategy so challenging is that it requires not only simulating differing navigational paths but also simulating your own future needs. Both a carnivore and a non-fruit-eating herbivore can survive by hunting or grazing only when they are hungry. But a frugivore must plan its trips in advance before it is hungry. Setting up camp en route to a nearby popular fruit patch the night before requires anticipating the fact that you will be hungry tomorrow if you don’t take preemptive steps tonight to get to the food early.
The Bischof-Kohler Hypothesis
In the 1970s, two comparative psychologists by the name of Doris Bischof-Kohler and her husband, Norbert Bischof, proposed a novel hypothesis about what was unique about planning in humans: They hypothesized that while other animals can make plans based on current needs (like how to get to food when they are hungry), only humans can make plans based on future needs (like how to get food for your trip next week, even though you are not hungry right now). The evolutionary psychologist Thomas Suddendorf would later call this the “Bischof-Kohler hypothesis.”
But Naqshbandi and Roberts then tested these animals in a different condition. Dates and raisins induce large amounts of thirst in these animals, often requiring them to consume over twice as much water to rehydrate themselves. So what happens if these animals are forced to make a trade-off, incorporating their future state of thirst? Naqshbani and Roberts modified the test such that if animals select the high treat option (the cup with many dates or raisins), they will only get access to water hours later; however, if animals select the low treat option (the cup with few dates or raisins) they get access to water between 15 and 30 minutes later. What happens?
This suggests that perhaps Suddendorf’s Bischof-Kohler hypothesis was correct that anticipating a future need is a more difficult form of planning and was correct that some animals should be able to plan but unable to anticipate future needs (such as rats). But it may not be the case that only humans were endowed with this ability. It may instead be the province of many primates.
How Primates Anticipate Future Needs
The mechanics of making a choice based on an anticipated need, one you are not currently experiencing, presents a predicament to the older mammalian brain structures. We have speculated that the mechanism by which the neocortex controls behavior is by simulating decisions vicariously, the outcomes of which are then evaluated by the older vertebrate structures (basal ganglia, amygdala, and hypothalamus). This mechanism allows an animal to choose only simulated paths and behaviors that excite positive valence neurons right now, like imagining food when hungry or water when thirsty.
In contrast, to buy groceries for the week, I need to anticipate a pizza is going to make a great addition to Thursday’s movie night, even though I don’t currently want pizza. When I imagine eating pizza while I’m not hungry, my basal ganglia doesn’t get excited; it doesn’t accumulate votes for any decisions to pursue pizza. Thus, to want pizza, I need to realize that in this imagined future state of hunger, the smell and sight of food will excite positive valence neurons, even though imagining it right now does not. How, then, can a brain choose an imagined path in the absence of any vicarious positive-valence activation? How can your neocortex want something that your amygdala and hypothalamus do not?
There is another situation we have already seen where brains need to infer an intent—a “want”—of which it does not currently share: when they’re trying to infer the wants of other people. Might brains be able to use the same mechanism of theory of mind to anticipate a future need? Put another way: Is imagining the mind of someone else really any different from imagining the mind of your future self?
Perhaps the mechanism by which we anticipate future needs is the same mechanism by which we engage in theory of mind: We can infer the intent of a mind—whether our own or someone else’s—in some different situation from our current one. Just as we can correctly infer the cravings of someone deprived of food (“How hungry would James be if he didn’t eat for twenty-four hours?”) even though we ourselves might not be hungry, perhaps too we can infer the intent of ourselves in a future situation (“How hungry would I be if I didn’t eat for twenty-four hours?”) even though we are currently not hungry.
In his paper discussing the Bischof-Kohler hypothesis, Thomas Suddendorf brilliantly foreshadowed exactly this idea:
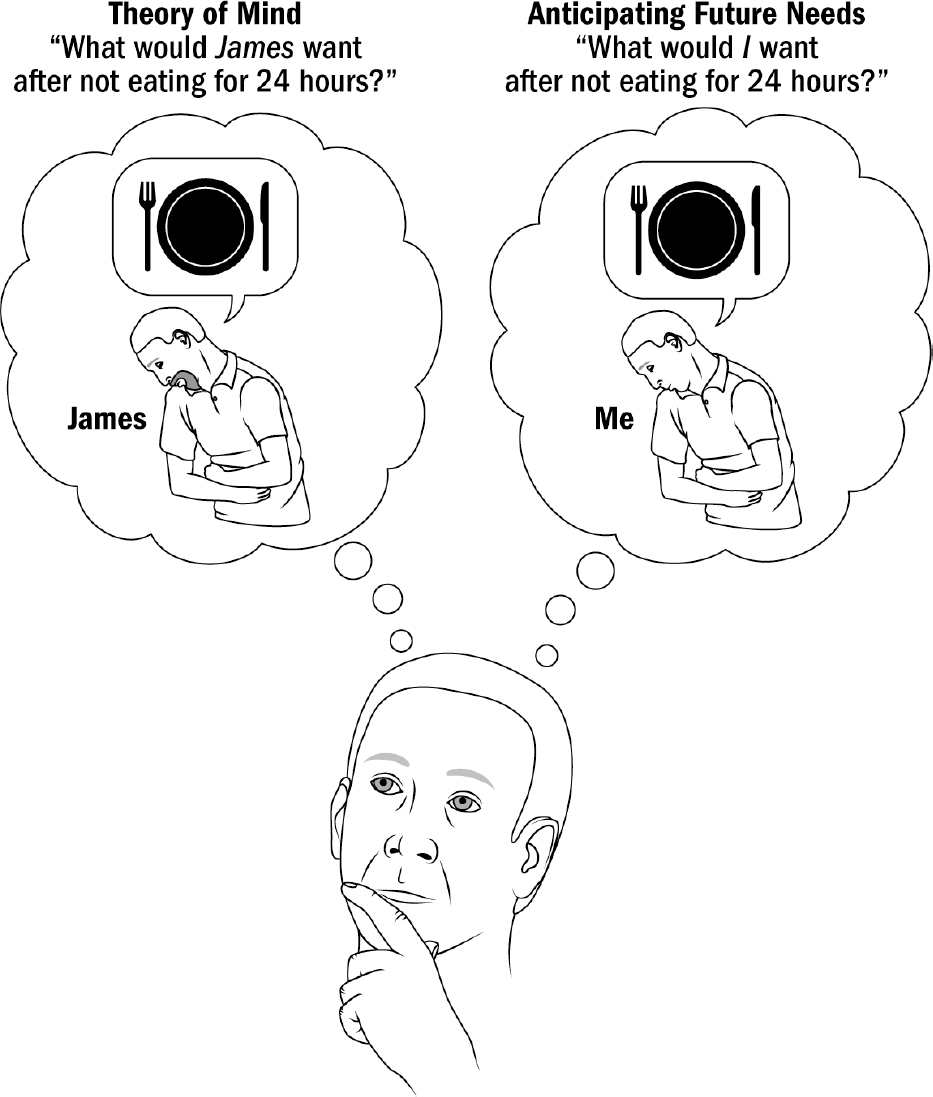
Figure 18.1: The similarity between theory of mind and anticipating future needs
Original art by Rebecca Gelernter
Future need anticipation . . . might be only a special case of animals’ general problem with simultaneously representing conflicting mental states. Like 3-year-old children, they may be unable to imagine an earlier belief (or state of knowledge, or drive, etc.) that is different from a present one or to understand that another individual holds a belief different from their own. This may apply to future states as well as to past ones. That is, a satiated animal may be unable to understand that it may later be hungry, and therefore may be unable to take steps to ensure that this future
There are two observations that support this idea. First, it seems that both theory of mind and anticipating future needs are present, even in a primitive form, in primates, but not in many other mammals, suggesting both abilities emerged around the same time in early primates. Second, people make similar types of mistakes in tasks of theory of mind and of anticipating future needs.
The ability to anticipate future needs would have offered numerous benefits to our ancestral frugivores. It would have enabled our ancestors to plan their foraging routes long in advance, thereby ensuring they were the first to get newly ripened fruits. Our ability to make decisions today for faraway, abstract, and not-yet-existent goals was inherited from tree faring primates. A trick that, perhaps, was first used for getting the first pick of fruits, but today, in humans, is used for far greater purposes. It laid the foundation for our ability to make long term plans over vast stretches of time.
Summary of Breakthrough #4: Mentalizing
There are three broad abilities that seem to have emerged in early primates:
- Theory of mind: inferring intent and knowledge of others
- Imitation learning: acquiring novel skills through observation
- Anticipating future needs: taking an action now to satisfy a want in the future, even though I do not want it now
These may not, in fact, have been separate abilities but rather emergent properties of a single new breakthrough: the construction of a generative model of one’s own mind, a trick that can be called “mentalizing.” We see this in the fact that these abilities emerge from shared neural structures (such as the gPFC) that evolved first in early primates. We see this in the fact that children seem to acquire these abilities
And most important, we see this in the fact that the structures from which these skills emerge are the same areas from which our ability to reason about our own mind emerges. These new primate areas are required not only for simulating the mind of others but also for projecting yourself into your imagined futures, identifying yourself in the mirror (mirror-sign syndrome), and identifying your own movements (alien-hand syndrome). And a child’s ability to reason about her own mind tends to precede a child’s development of all three of these abilities.
However, the best evidence for this idea goes all the way back to Mountcastle. The main change to the brain of early primates, besides its size, was the addition of new areas of neocortex. So if we are to stick to the general idea—inspired by Mountcastle, Helmholtz, Hinton, Hawkins, Friston, and many others—that every area of neocortex is made up of identical microcircuits, this imposes strict constraints on how we explain the newfound abilities of primates. It suggests that these new intellectual skills must emerge from some new clever application of the neocortex and not some novel computational trick. This makes the interpretation of theory of mind, imitation learning, and anticipation of future needs as nothing more than an emergent property of a second-order generative model a nice proposal—all three abilities can emerge from nothing more than new applications of neocortex.
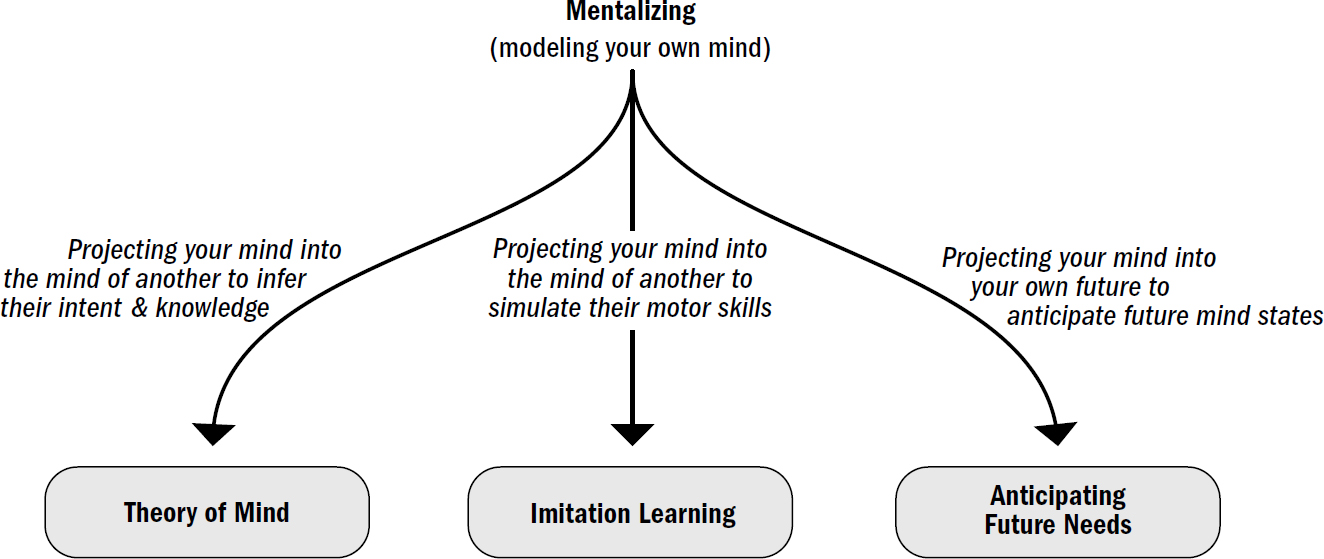
Figure 18.2
Original art by Rebecca Gelernter
All these abilities—theory of mind, imitation learning, and anticipating future needs—would have been particularly adaptive in the unique niche of early primates. Dunbar argues that the social-brain hypothesis and the ecological-brain hypothesis are two sides of the same coin. The ability to mentalize may have simultaneously unlocked both the ability to successfully forage fruits and to successfully politick. The pressures of both frugivorism and social hierarchies may have converged to produce continual evolutionary pressure to develop and elaborate brain regions—such as the gPFC—for modeling your own mind.
If we were to scrunch the six hundred million years of evolutionary time—from which the first brains emerged until today—into a single calendar year, then we would now find ourselves perched at Christmas Eve, the final seven days of December. Over the next “seven days,” our ancestors will go from foraging fruits to flying Falcon 9 rockets. Let’s find out how.
Breakthrough #5
Speaking and the First Humans

Your brain 100,000 years ago
Original art by Rebecca Gelernter
19
The Search for Human Uniqueness
FOR MILLENNIA, WE HUMANS have looked at ourselves in the mirror with self-congratulatory pride and pondered the many ways in which we are superior to our fellow animals. Aristotle claimed it was our “rational soul”—our ability to reason, make abstractions, and reflect—that was uniquely human. Twentieth-century animal psychologists itemized many intellectual abilities they believed were uniquely human. Some argued that only humans engage in mental time travel. Others, that it is our episodic memory. Others, our ability to anticipate future needs. Others, our sense of self. Our ability to communicate, coordinate, use of tools. The lists went on.
But the last century of research into the behaviors of other animals has methodically dismantled our surprisingly fragile edifice of uniqueness. Despite the intuitive appeal of claiming many of these skills as only ours, as we have seen throughout this book, science suggests many of them, if not all, might not be unique to humans at all.
Darwin believed that “the difference in mind between man and the higher animals, great as it is, is certainly one of degree
An eminently reasonable explanation of human brain evolution since our divergence with chimpanzees is that various evolutionary pressures led our human ancestors to simply “level up” the abilities that were already present.
Was there perhaps no breakthrough at all?
This seems to be the most reasonable interpretation—but for one crucial exception. And it is in this singular exception that we see the first hints of what it means to be human.
Our Unique Communication
Organisms had been communicating with each other long before early humans uttered their first words. Single-celled organisms emit chemical signals to share genes and information about the environment. Brainless sea anemones pump pheromones into the water to coordinate the timing of sperm and egg release. Bees dance to signal where to find food. Fish use electrical signals to court each other. Reptiles head-bob to communicate their aggressiveness. Mice squeak to express danger or excitement. Communication between organisms is evolutionarily ancient and ubiquitous.
Vervet monkeys have different sounds to signal the presence of specific predators. When one monkey makes the squeal meaning “Leopard!” all the others run to the trees. When one makes the squeal meaning “Eagle!” all the others jump to the forest floor. Experimenters can get all the monkeys to run to the treetops or jump to the floor simply by playing one of these sounds from nearby loudspeakers.
And then, of course, there is us—Homo sapiens. We too communicate with each other. It is not the fact that we communicate that is unique; rather, it is how we communicate. Humans use language.
Human language differs from other forms of animal communication in two ways. First, no other known form of naturally occurring animal communication assigns declarative labels (otherwise known as symbols). A human teacher will point to an object or a behavior and assign it an arbitrary label: elephant, tree, running. In contrast, other animals’ communications are genetically hardwired and not assigned. Vervet monkey and chimpanzee gestures are almost identical across different groups that have no contact with each other. Monkeys and apes deprived of social contact still use the same gestures. In fact, these gestures are even shared across species of primates; bonobos and chimpanzees share almost exactly the same repertoire of gestures and vocalizations. In nonhuman primates, the meanings of these gestures and vocalizations is not assigned through declarative labeling but emerge directly from genetic hardwiring.
What about teaching a dog or any other animal a command? Clearly this represents some form of labeling. Linguists make a distinction between declarative and imperative labels. An imperative label is one that yields a reward: “When I hear sit, if I sit, I will get a treat” or “When I hear stay, if I stop moving, I will get a treat.” This is basic temporal difference learning—all vertebrates can do this. Declarative labeling, on the other hand, is a special feature of human language. A declarative label is one that assigns an object or behavior an arbitrary symbol—“That is a cow,” “That is running,”—without any imperative at all. No other form of naturally occurring animal communication has been found to do this.
The second way in which human language differs from other animal communication is that it contains grammar. Human language contains rules by which we merge and modify symbols to convey specific meanings. We can thereby weave these declarative labels into sentences, and we can knit these sentences into concepts and stories. This allows us to convert the few thousand words present in a typical human language into a seemingly infinite number of unique meanings.
The simplest aspect of grammar is that the order in which we utter symbols conveys meaning: “Ben hugged James” means something different from “James hugged Ben.” We also embed subphrases that are sensitive to ordering: “Ben, who was sad, hugged James” means something entirely different than “Ben hugged James, who was sad.” But the rules of grammar go beyond just order. We have different tenses to convey timing: “Ben is attacking me” versus “Max attacked me.” We have different articles: “The pet barked” means something different than “A pet barked.”
And of course, this is just English; there are over six thousand spoken languages on Earth, each with its own labels and grammar. But despite the great diversity of the specific labels and grammars of different languages, every single group of humans ever discovered used language. Even hunter-gatherer societies in Australia and Africa who at the point they were “discovered” had had no contact with any other groups of humans for over fifty thousand years, still spoke their own languages that were equally complex as those of other humans. This is irrefutable evidence that the shared ancestor of humans spoke their own languages, with their own declarative labels and grammars.
Of course, the fact that early humans spoke in their own languages with declarative labels and grammar, while no other animal naturally does so, does not prove that only humans are able to use language, merely that only humans happen to use language. Did the brains of early humans really evolve some unique ability to speak? Or is language just a cultural trick that was discovered over fifty thousand years ago and was simply passed down through generations of all modern humans? Is language an evolutionary invention or a cultural invention?
Here’s one way to test this: What happens if we try to teach language to our evolutionarily closest animal cousins, our fellow apes? If apes succeed in learning language, that suggests that language was a cultural invention; if apes fail, this suggests their brains lack a key evolutionary innovation that emerged in humans.
This test has been performed multiple times. The result is as surprising as it is revealing.
Attempts to Teach Apes Language
To start: We can’t literally teach apes to speak. This was attempted in the 1930s, and it failed—nonhuman apes are physically incapable of producing verbal language. Human vocal cords are uniquely adapted to speech; the human larynx is lower and the human neck is longer, which enables us to produce a much wider variety of vowels and consonants than other apes. The vocal cords of a chimp can produce only a limited repertoire of huffs and squeals.
However, what makes language language is not the medium but the substance—many forms of human language are nonverbal. No one would claim that writing, sign language, and Braille do not contain the substance of language because they don’t involve vocalization.
The key studies that attempted to teach chimpanzees, gorillas, and bonobos language used either American Sign Language or made-up visual languages in which apes pointed to sequences of symbols on a board. Beginning as infants, these apes were trained to use these languages, with human experimenters signing or pointing to symbols to refer to objects (apples, bananas) or actions (tickling, playing, chasing) over and over again until the apes began to repeat the symbols.
Across most of these studies, after years of being taught, nonhuman apes did indeed produce the appropriate signs. They could look at a dog and sign dog and look at a shoe and sign shoe.
On balance, most scientists seem to conclude that some nonhuman apes are indeed capable of learning at least a rudimentary form of language but that nonhuman apes are much worse than humans at it and don’t learn it without painstaking deliberate training. These apes never surpass the abilities of a young human child.
So, language seems unique to humans on two counts. First, we have a natural tendency to construct it and use it, which other animals do not. Second, we have a capacity for language that far surpasses that of any other animal, even if some basic semblance of symbols and grammar is possible in other apes.
But if language is what separates us from the rest of the animal kingdom, then what is it about this seemingly innocuous trick that enabled Homo sapiens to ascend to the top of food chain; what is it about language that makes those who wield it so powerful?
Transferring Thoughts
When we talk about these inner simulations, especially in the context of humans, we tend to imbue them with words like concepts, ideas, thoughts. But all these things are nothing more than renderings in the mammalian neocortical simulation. When you “think” about a past or future event, when you ponder the “concept” of a bird, when you have an “idea” as to how to make a new tool, you are merely exploring the rich three-dimensional simulated world constructed by your neocortex. It is no different, in principle, than a mouse considering which direction to turn in a maze. Concepts, ideas, and thoughts, just like episodic memories and plans, are not unique to humans. What is unique is our ability to deliberately transfer these inner simulations to each other, a trick possible only because of language.
When a vervet monkey makes an Eagle nearby! squeal, all nearby monkeys will quickly jump from the trees to hide. Clearly, this represents a transfer of information from the monkey who first saw the eagle to the others. But these kinds of transfers are undetailed and inflexible, capable of transferring information only with genetically hard-coded signals. These signals are always few in number and cannot be adjusted or changed to new situations. In contrast, language enables the talker to transfer an incredibly broad set of inner thoughts.
This trick of thought transfer would have provided many practical benefits to early humans. It would have enabled more accurate teaching of tool use, hunting techniques, and foraging tricks. It would have enabled flexible coordination of scavenging and hunting behaviors across individuals—a human could say, “Follow me, there is an antelope carcass two miles east” or “Wait here, let’s ambush the antelope when you hear me whistle three times.”
All these practical benefits emerge from the fact that language expands the scope of sources a brain can extract learnings from. The breakthrough of reinforcing enabled early vertebrates to learn from their own actual actions (trial and error). The breakthrough of simulating enabled early mammals to learn from their own imagined actions (vicarious trial and error). The breakthrough of mentalizing enabled early primates to learn from other people’s actual actions (imitation learning). But the breakthrough of speaking uniquely enabled early humans to learn from other people’s imagined actions.
The Evolution of Progressively More Complex Sources of Learning
REINFORCING IN EARLY BILATERIANS | SIMULATING IN EARLY VERTEBRATES | MENTALIZING IN EARLY PRIMATES | SPEAKING IN EARLY HUMANS | |
SOURCE OF LEARNING | Learning from your own actual actions | Learning from your own imagined actions | Learning from others’ actual actions | Learning from others’ imagined actions |
WHO LEARNING FROM? | Yourself | Yourself | Others | Others |
ACTION LEARNING FROM? | Actual actions | Imagined actions | Actual actions | Imagined actions |
Language enables us to peer into and learn from the imagination of other minds—from their episodic memories, their internal simulated future actions, their counterfactuals. When a human coordinates a hunt and says, “If we go in this direction as a group we will find an antelope” or “If we all wait and ambush we will win the battle with the boar,” humans are sharing the outcomes of their own inner vicarious trial and errors so that the whole group can learn from their imaginations. One person with an episodic memory of a lion on the other side of a mountain can transfer that episodic memory to others with language.
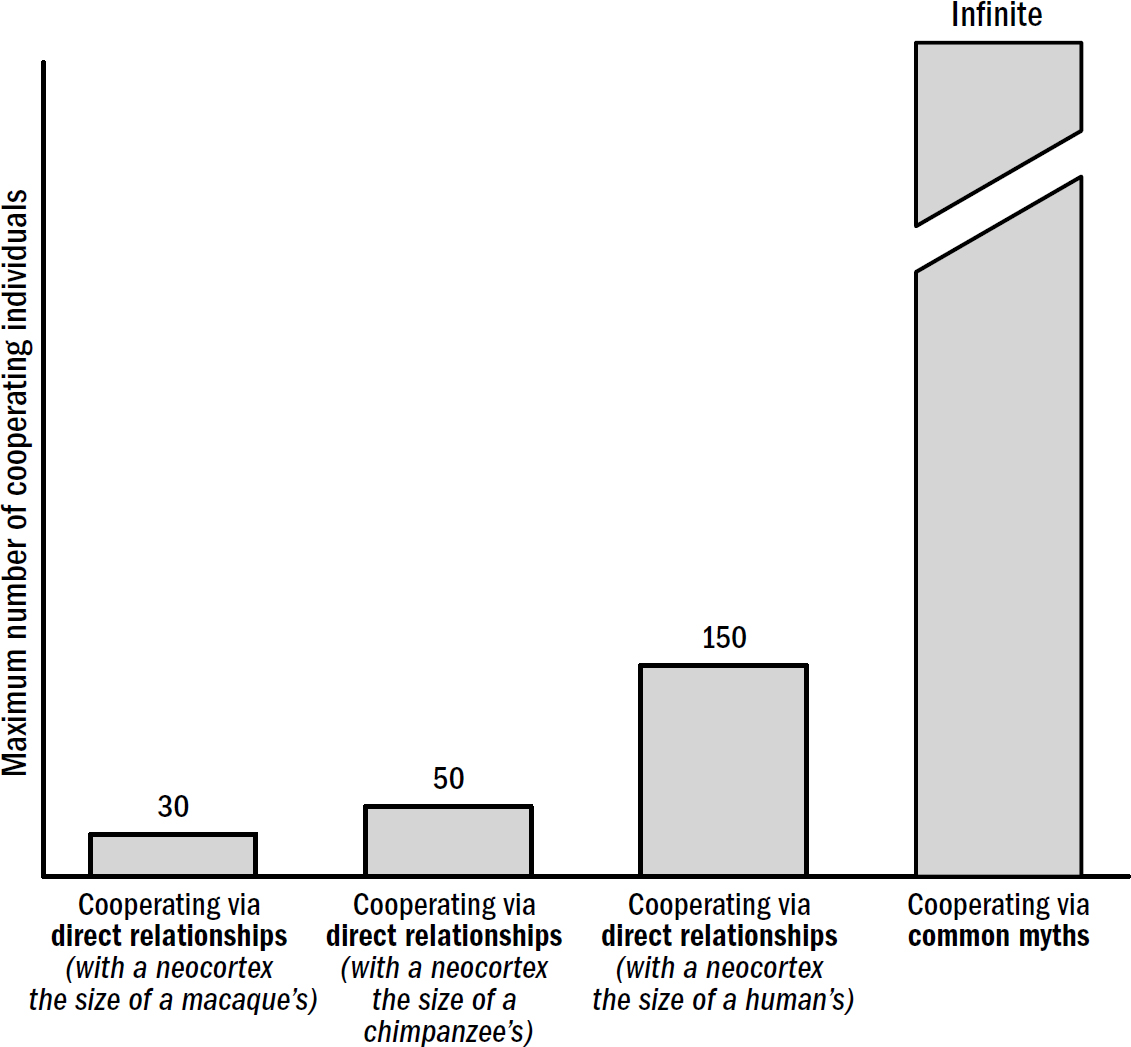
Original art by Rebecca Gelernter
While true, all these aforementioned benefits of language miss the larger point. It is not superior teaching, cooperative hunting, or Harari’s common myths that is the true gift of language. None of these are why humans rule the world. If these were the only gifts language offered, we would still be hunter-gatherer apes dancing around campfires praying for rain from the water gods—apex predators, sure, but hardly astronauts. These features of language are consequences of the gift of language, not the gift itself.
An analogy to DNA is useful. The true power of DNA is not the products it constructs (hearts, livers, brains) but the process it enables (evolution). In this same way, the power of language is not its products (better teaching, coordinating, and common myths) but the process of ideas being transferred, accumulated, and modified across generations. Just as genes persist by hopping from parent cell to offspring cell, ideas persist by hopping from brain to brain, from generation to generation. And as with genes, this hopping is not uniform but operates under its own quasi-evolutionary rules—there is a continual selecting of good ideas and pruning of bad ideas. Ideas that helped humans survive persisted, while those that did not perished.
This analogy of ideas evolving was proposed by Richard Dawkins in his famous book The Selfish Gene. He called these hopping ideas memes. This word was later appropriated for cat images and baby photos flying around Twitter, but he originally meant them to refer to an idea or behavior that spread from person to person in a culture.
This accumulation does not apply only to technological inventions but also to cultural ones. We pass down social etiquette, values, stories, mechanisms for selecting leaders, moral rules of punishment, and cultural beliefs around violence and forgiveness.
All human inventions, both technological and cultural, require an accumulation of basic building blocks before a single inventor can go “Aha!,” merge the preexisting ideas into something new, and transfer this new invention to others. If the baseline of ideas always fades after a generation or two, then a species will be forever stuck in a nonaccumulating state, always reinventing the same ideas over and over again. This is how it is for all other creatures in the animal kingdom. Even chimpanzees, who learn motor skills through observation, do not accumulate learnings across generations.
While these imitation experiments demonstrate that humans can accurately copy behaviors without using language, it is still undeniably language that is our superpower in the business of copying and transferring ideas.
The Singularity Already Happened
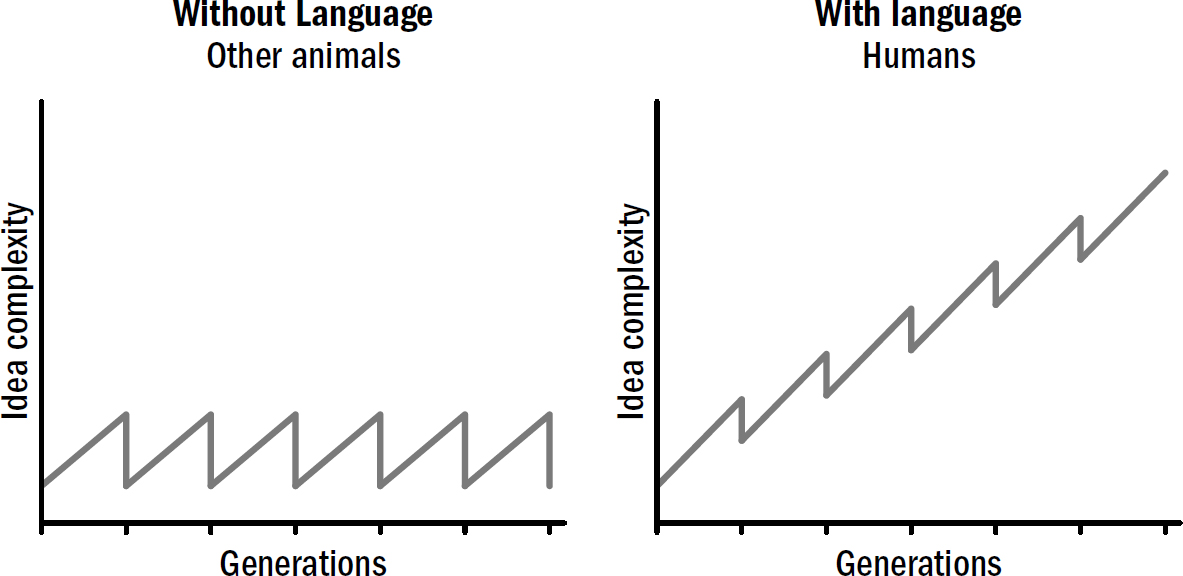
Figure 19.2
Original art by Rebecca Gelernter
And if you zoom out to the timescale of thousands of generations, you see why even just some accumulation triggers an explosion of idea complexity (as seen in figure 19.3). From a period of seemingly perpetual stasis, you will, in a matter of a few hundred thousand years, get an explosion of complex ideas.
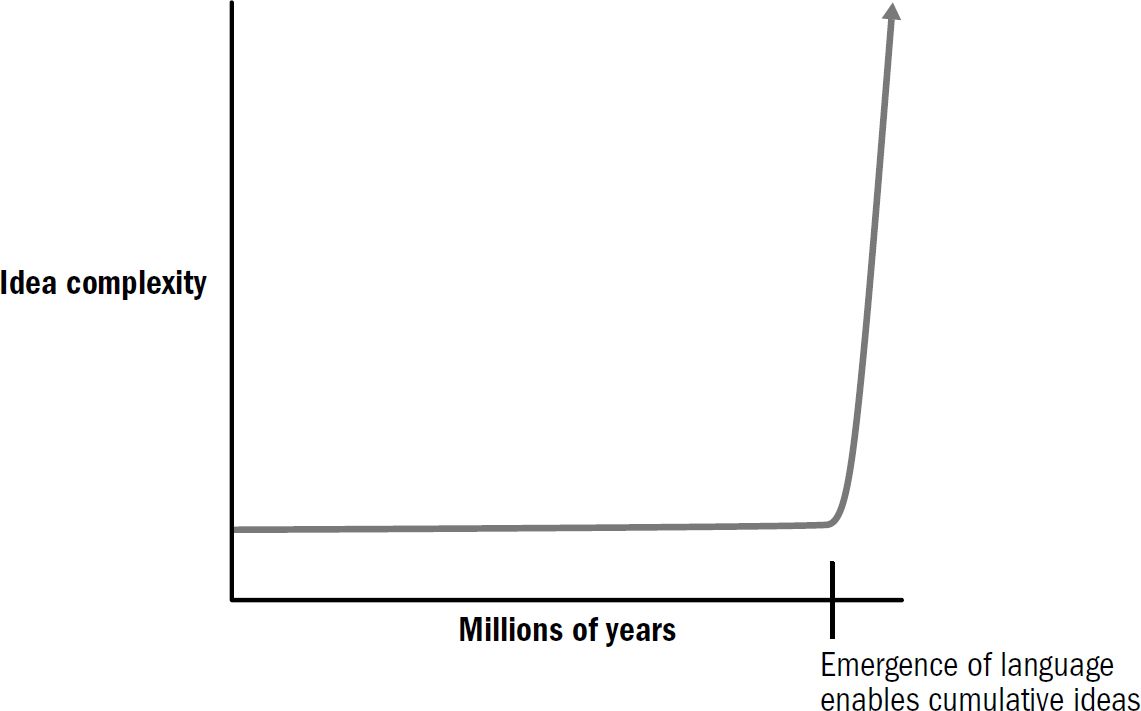
Figure 19.3
Original art by Rebecca Gelernter
Eventually, the corpus of ideas accumulated reached a tipping point of complexity when the total sum of accumulated ideas no longer fit into the brain of a single human. This created a problem in sufficiently copying ideas across generations. In response, four things happened that further expanded the extent of knowledge that could be transferred across generations. First, humans evolved bigger brains, which increased the amount of knowledge that can be passed down through individual brains. Second, humans became more specialized within their groups, with ideas distributed across different members—some were the spear makers, others clothing makers, others hunters, others foragers. Third, population sizes expanded, which offered more brains to store ideas across generations. And fourth, most recent and most important, we invented writing. Writing allows humans to have a collective memory of ideas that can be downloaded at will and that can contain effectively an infinite corpus of knowledge.
If groups don’t have writing, such distributed knowledge is sensitive to group size; if groups shrink, and there are no longer enough brains to fit all the information into, knowledge is lost. There is evidence that this occurred in societies in Tasmania. Archaeological evidence from eight thousand years ago shows that humans in Tasmania had complex knowledge of making bone tools, nets, fishing spears, boomerangs, and cold-weather clothing. All this knowledge was lost by the 1800s. This loss seems to have been initiated when rising oceans cut the group of humans in Tasmania off from other groups in the rest of Australia, effectively lowering the population size of the group of socially interacting humans. For people without writing, the smaller the population, the less knowledge
The real reason why humans are unique is that we accumulate our shared simulations (ideas, knowledge, concepts, thoughts) across generations. We are the hive-brain apes. We synchronize our inner simulations, turning human cultures into a kind of meta-life-form whose consciousness is instantiated within the persistent ideas and thoughts flowing through millions of human brains over generations. The bedrock of this hive brain is our language.
The emergence of language marked an inflection point in humanity’s history, the temporal boundary when this new and unique kind of evolution began: the evolution of ideas. In this way, the emergence of language was as monumental an event as the emergence of the first self-replicating DNA molecules. Language transformed the human brain from an ephemeral organ to an eternal medium of accumulating inventions.
These inventions included new technologies, new laws, new social etiquettes, new ways of thinking, new systems of coordination, new ways of selecting leaders, new thresholds for violence versus forgiveness, new values, new shared fictions. The neurological mechanisms that enable language came far before anyone was doing math, using computers, or discussing the merits of capitalism. But once humans were armed with language, these developments were all but inevitable. It was just a matter of time. Indeed, the incredible ascent of humankind during the past few thousand years had nothing to do with better genes and everything to do with the accumulation of better and more sophisticated ideas.
20
Language in the Brain
IN 1830, A THIRTY-YEAR-OLD Frenchman by the name of Louis Victor Leborgne lost the ability to speak. Leborgne could no longer say anything other than the syllable tan. What was peculiar about Leborgne’s case was that he was, for the most part, otherwise intellectually typical. It was clear that when he spoke, he was trying to express certain ideas—he would use gestures and alter the tone and emphasis of his speech—but the only sound that ever came out was tan. Leborgne could understand language; he just couldn’t produce it. After many years of hospitalization, he became known around the hospital as Tan.
Twenty years after patient Tan passed away, his brain was examined by a French physician named Paul Broca who had a particular interest in the neurology of language. Broca found that Leborgne had brain damage to a specific and isolated region in the left frontal lobe.
Broca had a hunch that there were specific areas in the brain for language. Leborgne’s brain was Broca’s first clue that this idea might be right. Over the next two years, Broca painstakingly sought out the brains of any recently deceased patients who had had impairment in their ability to articulate language but retained their other intellectual faculties. In 1865, after performing autopsies on twelve different brains, he published his now famous paper “Localization of Speech in the Third Left Frontal Cultivation.” It turned out that all of these patients had damage to similar regions on the left side of the neocortex, a region that has come to be called Broca’s area. This has been observed countless times over the past hundred and fifty years—if Broca’s area is damaged, humans lose the ability to produce speech, a condition now called Broca’s aphasia.
Several years after Broca did his work, Carl Wernicke, a German physician, was perplexed by a different set of language difficulties. Wernicke found patients who, unlike Broca’s, could speak fine but lacked the ability
Wernicke, following Broca’s strategy, also found a damaged area in the brains of these patients. It was also on the left side but farther back in the posterior neocortex, a region now dubbed Wernicke’s area. Damage to Wernicke’s causes Wernicke’s aphasia, a condition in which patients lose the ability to understand speech.
Figure 20.1
Original art by Rebecca Gelernter
The human motor cortex has a unique connection directly to the brainstem area for controlling the larynx and vocal cords—this is one of the few structural differences between the brains of humans and those of other apes. The human neocortex can uniquely control the vocal cords, which is surely an adaptation for using verbal language. But this is a red herring in trying to understand the evolution of language; this unique circuitry is not the evolutionary breakthrough that enabled language. We know this because humans can learn nonverbal language with as much fluency and ease as they learn verbal language—language is not a trick that requires this wiring with the vocal cords. Humans’ unique control of the larynx either coevolved with other changes for language in general, evolved after them (to transition from a gesture-like language to a verbal language), or evolved before them (adapted for some other nonlanguage purpose). In any case, it is not human control of the larynx that enabled language.
This suggests that language is not an inevitable consequence of having more neocortex. It is not something humans got “for free” by virtue of scaling up a chimpanzee brain. Language is a specific and independent skill that evolution wove into our brains.
So this would seem to close the case. We have found the language organ of the human brain: humans evolved two new areas of neocortex—Broca’s and Wernicke’s areas—which are wired together into a specific subnetwork specialized for language. This subnetwork gifted us language, and that is why humans have language and other apes don’t. Case closed.
Unfortunately, the story is not so simple.
Laughs or Language?
Perhaps human language was an elaboration on the existing system of ape communication? This might explain why these language areas are still present in other primates. Chimpanzees, bonobos, and gorillas all have sophisticated suites of gestures and hoots that signal different things. Wings evolved from arms, and multicellular organisms evolved from single-celled organisms, so it would make sense if human language evolved from the more primitive communication systems of our ape ancestors. But this is not how language evolved in the brain.
When we compare ape gestures to human language, we are comparing apples to oranges. Their common use for communication obscures the fact that they are entirely different neurological systems without any evolutionary relationship to each other.
Humans have, in fact, inherited the exact same communication system of apes, but it isn’t our language—it is our emotional expressions.
When examining the man, the doctor noticed something perplexing. When the doctor told a joke or said something genuinely pleasant, the man could smile just fine. The left side of his face worked normally when he was laughing, but when he was asked to smile voluntarily, the man was unable to do it.
The human brain has parallel control of facial expressions; there is an older emotional-expression system that has a hard-coded mapping between emotional states and reflexive responses. This system is controlled by ancient structures like the amygdala. Then there is a separate system that provides voluntary control of facial muscles that is

Images from Trepel et al., 1996. Used with permission.
It turned out that this teacher had a lesion in his brain stem that had disrupted the connection between his neocortex and the muscles on the left side of his face but had spared the connection between his amygdala and those same muscles. This meant that he couldn’t voluntarily control the left side of his face, but his emotional-expression system could control his face just fine. While he was unable to voluntarily lift an eyebrow, he was eminently able to laugh, frown, and cry.
This is also seen in individuals with severe forms of Broca’s and Wernicke’s aphasia. Even individuals who can’t utter a single word can readily laugh and cry. Why? Because emotional expressions emerge from a system entirely separate from language.
Figure 20.3
Original art by Rebecca Gelernter
Human laughs, cries, and scowls are evolutionary remnants of an ancient and more primitive system for communication, a system from which ape hoots and gestures emerge. However, when we speak words, we are doing something without any clear analog to any system of ape communication.
This explains why lesions to Broca’s and Wernicke’s areas in monkeys have absolutely no impact on communication. A monkey can still hoot and holler for the same reason a human with such damage can still laugh, cry, smile, frown, and scowl even while he can’t utter a single coherent word. The gestures of monkeys are automatic emotional expressions and don’t emerge from the neocortex; they are more like a human laugh than language.
So here is the neurobiological conundrum of language. Language did not emerge from some newly evolved structure. Language did not emerge from humans’ unique neocortical control over the larynx and face (although this did enable more complex verbalizations). Language did not emerge from some elaboration of the communication systems of early apes. And yet, language is entirely new.
So what unlocked language?
The Language Curriculum
All birds know how to fly. Does this mean that all birds have genetically hardwired knowledge of flying? Well, no. Birds are not born knowing how to fly; all baby birds must independently learn how to fly. They start by flapping wings, trying to hover, making their first glide attempt, and eventually, after enough repetitions, they figure it out. But if flying is not genetically hard-coded, then how is it that approximately 100 percent of all baby birds independently learn such a complex skill?
A skill as sophisticated as flying is too information-dense to hard-code directly into a genome. It is more efficient to encode a generic learning system (such as a cortex) and a specific hardwired learning curriculum (instinct to want to jump, instinct to flap wings, and instinct to attempt to glide). It is the pairing of a learning system and a curriculum that enables every single baby bird to learn how to fly.
It didn’t work.
Then Elman tried something different. Instead of showing the neural network sentences of all levels of complexity at the same time, he first showed it extremely simple sentences, and only after the network performed well at these did he increase the level of complexity. In other words, he designed a curriculum. And this, it turned out, worked. After being trained with this curriculum, his neural network could correctly complete complex sentences.
To teach a new skill, it is often easier to change the curriculum instead of changing the learning system. Indeed, this is the solution that evolution seems to have repeatedly settled on when enabling complex skills—monkey climbing, bird flying, and, yes, even human language all seem to work this way. They emerge from newly evolved hardwired curriculums.
What’s the point of children’s quirky prewired ability to engage in proto-conversations and joint attention? It is not for imitation learning; nonhuman primates engage in imitation learning just fine without proto-conversations or joint attention. It is not for building social bonds; nonhuman primates and other mammals have plenty of other mechanisms for building social bonds. It seems that joint attention and proto-conversations evolved for a single reason. What is one of the first things that parents do once they have achieved a state of joint attention with their child? They assign labels to things.
So we don’t realize it, but when we happily go back and forth making incoherent babbles with babies (proto-conversations), when we pass objects back and forth and smile (joint attention), and when we pose and answer even nonsensical questions from infants, we are unknowingly executing an evolutionarily hard-coded learning program designed to give human infants the gift of language. This is why humans deprived of contact with others will develop emotional expressions, but they’ll never develop language. The language curriculum requires both a teacher and a student.
And as this instinctual learning curriculum is executed, young human brains repurpose older mentalizing areas of the neocortex for the new purpose of language. It isn’t Broca’s or Wernicke’s areas that are new, it is the underlying learning program that repurposes them for language that is new. As proof that there is nothing special about Broca’s or Wernicke’s areas: Children with the entire left hemisphere removed can still learn language just fine and will repurpose other areas of the neocortex on the right side of the brain to execute language. In fact, about 10 percent of people, for whatever reason, tend to use the right side of the brain, not the left, for language. Newer studies are even calling into question the idea that Broca’s and Wernicke’s areas are actually the loci of language; language areas may be located all over the neocortex and even in the basal ganglia.
Here is the point: There is no language organ in the human brain, just as there is no flight organ in the bird brain. Asking where language lives in the brain may be as silly as asking where playing baseball or playing guitar lives in the brain. Such complex skills are not localized to a specific area; they emerge from a complex interplay of many areas. What makes these skills possible is not a single region that executes them but a curriculum that forces a complex network of regions to work together to learn them.
So this is why your brain and a chimp brain are practically identical and yet only humans have language. What is unique in the human brain is not in the neocortex; what is unique is hidden and subtle, tucked deep in older structures like the amygdala and brain stem. It is an adjustment to hardwired instincts that makes us take turns, makes children and parents stare back and forth, and that makes us ask questions.
This is also why apes can learn the basics of language. The ape neocortex is eminently capable of it. Apes struggle to become sophisticated at it merely because they don’t have the required instincts to learn it. It is hard to get chimps to engage in joint attention; it is hard to get them to take turns; and they have no instinct to share their thoughts or ask questions. And without these instincts, language is largely out of reach, just as a bird without the instinct to jump would never learn to fly.
So, to recap: We know that the breakthrough that makes the human brain different is that of language. It is powerful because it allows us to learn from other people’s imaginations and allows ideas to accumulate across generations. And we know that language emerges in the human brain through a hardwired curriculum to learn it that repurposes older mentalizing neocortical areas into language areas.
With this knowledge, we can now turn to the actual story of our ancestral early humans. We can ask: Why were ancestral humans endowed with this odd and specific form of communication? Or perhaps more important: Why were the many other smart animals—chimps, birds, whales—not endowed with this odd and specific form of communication? Most evolutionary tricks that are as powerful as language are independently found by multiple lineages; eyes, wings, and multicellularity all independently evolved multiple times. Indeed, simulation and perhaps even mentalizing seem to have independently evolved along other lineages (birds show signs of simulation, and other mammals outside of just primates show hints of theory of mind). And yet language, at least as far as we know, has emerged only once. Why?
21
The Perfect Storm
SUPPOSE YOU TOOK all the presently discovered adult fossilized skulls of our ancestors, carbon-dated them (which tells you approximately how long ago they died), and then measured the size of the spaces inside their skulls (a good proxy for the size of their brains). And then suppose you graphed the size of these ancestral brains over time. Scientists have done this, and what you get is figure 21.1.
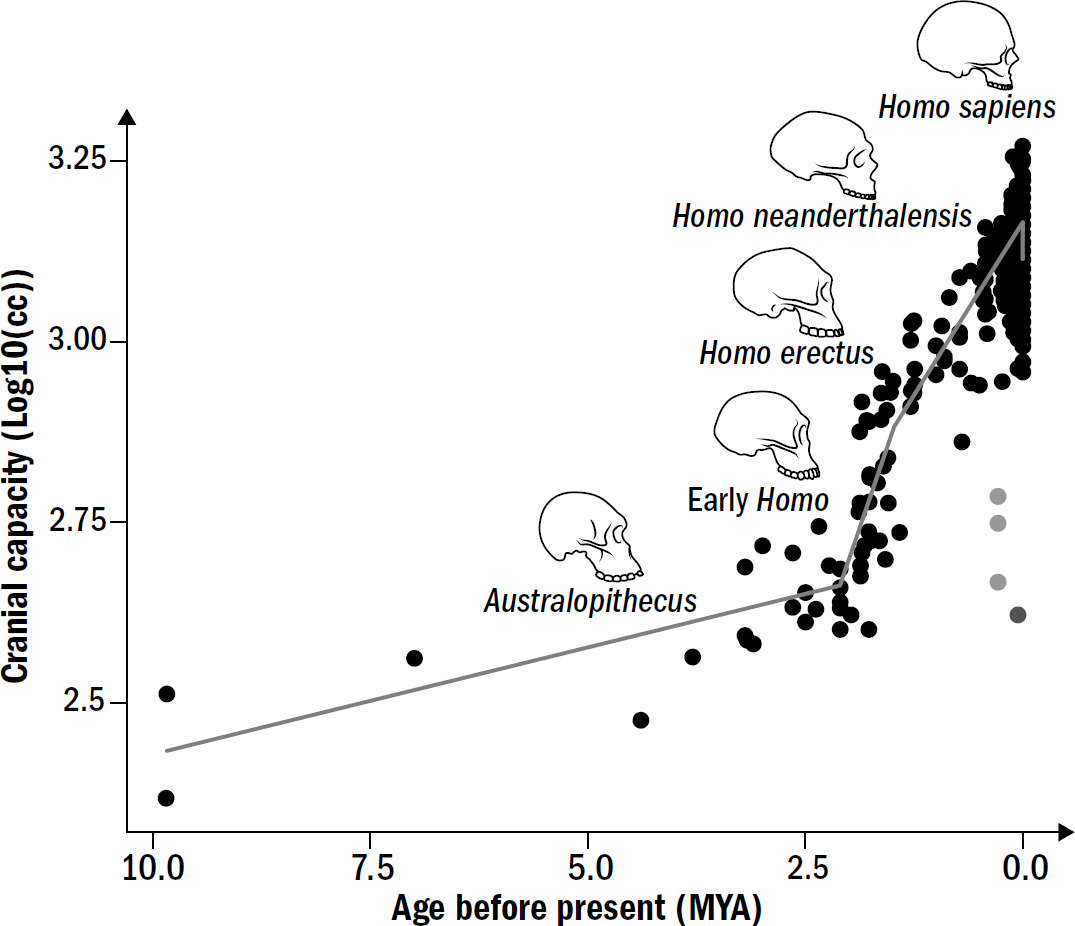
Original art by Rebecca Gelernter
Why exactly this happened is an outstanding question in paleoanthropology. We have only sparse archaeological clues: smatterings of ancient tools, hints of campfires, ancestral skull fragments, remnants of hunted carcasses, snippets of DNA, cave paintings, and broken pieces of prehistoric jewelry. Our understanding of the timeline of events changes with each new archaeological finding. The earliest known evidence of [X] is only the earliest until a new ambitious paleoanthropologist uncovers an even earlier sample. But despite this shifting timeline, there is still more than enough evidence for scientists to reconstruct the basics of our general story. It begins with a dying forest.
The East Side Ape
Until ten million years ago, eastern Africa was an arboreal oasis, endless acres of densely packed trees in which our ancestors could forage fruit and hide from predators. Then shifting tectonic plates began squeezing huge chunks of earth together, constructing new terrain and mountain ranges down the length of today’s Ethiopia. This region is today named the Great Rift Valley.
Figure 21.2: The east side apes and the west side apes
Original art by Rebecca Gelernter
Homo Erectus and the Rise of Humans
By two and a half million years ago, the new African savannah had become heavily populated with massive herbivorous mammals; ancestral elephants, zebras, giraffes, and hogs wandered and grazed. The savannah also became home to diverse populations of carnivorous mammals, familiar hunters like leopards, lions, and hyenas along with a cast of now extinct animals like saber-toothed tigers and gargantuan otter-like beasts.
And amid this cacophonous zoo of large mammals was a humble ape who had been displaced from its comfortable forest habitat. And this humble ape—our ancestor—would have been searching for a new survival niche in this ecosystem brimming with armies of giant herbivores and carnivorous hunters.
We infer this scavenging lifestyle from the tools and bone markings they left behind. These ancestors invented stone tools that seemed to be used specifically for processing the meat and bones of carcasses. These tools are referred to as “Oldowan tools” after the location where they were discovered (Olduvai Gorge in Tanzania).
Our ancestors constructed these tools in three steps: (1) They found a hammerstone made of hard rock; (2) they found a core made of more fragile quartz, obsidian, or basalt; (3) they smashed the hammerstone against the core to produce multiple sharp flakes and a pointed chopper.
Ape bodies aren’t adapted for consuming large quantities of meat; while lions can use their massive teeth to slice through thick hides and rip meat off bones, our ancestors had no such natural tools. So our ancestors invented artificial tools. Stone flakes could slice through hides and cut away meat, and stone choppers could smash open bones to access nutritious marrow.
Figure 21.3: Manufacture of Oldowan tools
Original art by Rebecca Gelernter
Homo erectus also evolved adaptations for endurance running. Legs elongated, feet became more arched, skin became hairless, and sweat glands proliferated. Both Homo erectus and modern humans have a peculiar method of cooling down—while other mammals pant to lower their body temperature, modern humans sweat. These traits would have kept our ancestors’ bodies cool while they were trekking long distances in the hot savannah. While modern humans are hardly the fastest creatures, we are actually some of the best endurance runners in the animal kingdom; even a cheetah couldn’t run a twenty-six-mile marathon in one go. Some believe H. erectus used a technique called persistence hunting—chasing prey until it was simply too tired to go any farther. This is exactly the technique used by modern hunter-gatherers in the Kalahari Desert of southern Africa.
Another unique feature of human brain development, in addition to how premature brains are at birth, is how long it takes for human brains to reach their full adult size. Setting a record among even the smartest and biggest-brained animals in the animal kingdom, it takes a human brain twelve years before it has reached its full adult size.
SPECIES | PERCENT OF ADULT BRAIN SIZE AT BIRTH | TIME UNTIL FULL BRAIN SIZE ACHIEVED |
Human | 28 percent | 12 years |
Chimpanzee | 36 percent | 6 years |
Macaque | 70 percent | 3 years |
Homo erectus was our meat-eating, stone-tool-using, (possibly) fire-wielding, premature-birthing, (mostly) monogamous, grandmothering, hairless, sweating, big-brained ancestor. The million-dollar question is, of course, did Homo erectus speak?
Wallace’s Problem
Long before Darwin discovered evolution, people were pondering the origins of language. Plato considered it. The Bible describes it. Many of the Enlightenment intellectuals who contemplated humankind’s state of nature, from Jean-Jacques Rousseau to Thomas Hobbes, speculated about it.
Part of what makes answering these questions so difficult is that there are no examples of living species with only a little bit of language. Instead, there are nonhuman primates with no naturally occurring language and Homo sapiens with language. If any Neanderthals or members of Homo erectus had survived to this day, we might have far more clues as to the process by which language emerged. But all humans alive today descended from a common ancestor around one hundred thousand years ago. Our nearest living cousin is the chimp, with whom we share a common ancestor who lived over seven million years ago. The evolutionary cavern between these periods leaves us without any living species from which to decipher the intermediary stages of language evolution.
Adhering to these milestones, the modern stories of language evolution run the entire gamut of possibilities. Some argue basic protolanguages emerged two and a half million years ago with the very first humans before Homo erectus; others argue that it emerged as late as one hundred thousand years ago uniquely in Homo sapiens. Some argue that language evolution was gradual; others say it occurred rapidly and all at once. Some argue that language began gesturally; others say that it began verbally.
Figure 21.4: Clues for reconstructing the timeline of language evolution
Original art by Rebecca Gelernter
These debates often restate old ideas in new forms; in many ways, today’s stories of language evolution are just as speculative as they were when the French banned discussions of it over one hundred fifty years ago. But in other ways, things are different. We have a far greater understanding of behavior, brains, and the archaeological record. And perhaps most important, we have a far greater understanding of the machinations of evolution, and it is here where we find our greatest clue to the origin of language.
The Altruists
It is intuitive to argue that language should have evolved for the same reason as any other useful evolutionary adaptation. Take the eye. If human A had slightly better eyes than human B, then human A had a higher probability of successfully hunting and mating. Hence, over time, the better-eyes gene should propagate through the population.
There is a crucial difference, however, with language. Language doesn’t directly benefit an individual the way eyes do; it benefits individuals only if others are using language with them in a useful way.
Well, perhaps the same evolutionary logic that applies to individuals might apply to groups: If group A of humans evolved a little bit of language, and group B of humans had no language, then group A would survive better, hence any progressive improvements to language would be selected for.
While many modern biologists agree that such group-level effects do occur in evolution, these group-level effects are far more nuanced and complex than the simple selection of traits that support the survival of the species. Evolution does not work this way. The problem is that genes do not spontaneously appear in groups, they appear in individuals.
Suppose 10 percent of group A is altruistic—they freely share information, teach others how to use tools, and reveal the locations of food. And assume the other 90 percent is not altruistic—they don’t share locations of food or spend time teaching tool use. Why would this subgroup of altruists fare any better? Wouldn’t a freeloader who was happy to accept these learnings but gave nothing in return survive better than the altruists?
Altruism is not what biologists call an evolutionarily stable strategy. The strategy of violating, cheating, and freeloading seems to better serve the survival of one’s individual genes.
In all these situations, defecting hurts only yourself. A fish that decides to leave the shoal and swim on its own will be the first to be eaten. Same for a wildebeest. But language is not like this; defecting in language—directly lying or withholding information—has many benefits to an individual. And the presence of liars and cheaters defeats the value of language. In a group where everyone is lying to each other with words, those who spoke no language and were immune to the lies might in fact survive better than those with language. So the presence of language creates a niche for defectors, which eliminates the original value of language. How, then, could language ever propagate and persist within a group?
In this way, the fifth breakthrough in the evolution of the human brain—language—is unlike any other breakthrough chronicled in this book. Steering, reinforcing, simulating, and mentalizing were adaptations that clearly benefited any individual organisms in which they began to emerge, and thus the evolutionary machinations by which they propagated are straightforward. Language, however, is only valuable if a group of individuals are using it. And so more nuanced evolutionary machinations must have been at work.
There are two types of altruism found in the animal kingdom. The first is called kin selection. Kin selection is when individuals make personal sacrifices for the betterment of their directly related kin. A gene has two ways to persist: improve its host’s chance of survival or help the host’s siblings and children survive. A child and sibling both have a 50 percent chance of sharing one of your individual genes. A grandchild has a 25 percent chance. A cousin has a 12.5 percent chance. In the context of evolutionary pressures, there is literally a mathematical expression comparing the value an organism places on its own life relative to that of its relatives. As the evolutionary biologist J. B. S. Haldane famously quipped: “I would happily lay down my life for two brothers or eight cousins.” This is why many birds, mammals, fish, and insects make personal sacrifices for their offspring but much less so for cousins and strangers.
Much behavior of modern humans, however, doesn’t fit cleanly into kin selection or reciprocal altruism. Sure, humans are clearly biased toward their own kin. But people still regularly help strangers without expecting anything in return. We donate to charity; we are willing to go to war and risk our lives for our fellow citizens, most of whom we’ve never met; and we take part in social movements that don’t directly benefit us but help strangers we feel have been disadvantaged. Think about how weird it would be for a human to see a lost and scared child on the street and just do nothing. Most humans would stop to help a child and do so without expecting any reciprocity in return. Humans are, relative to other animals, by far the most altruistic to unrelated strangers.
Of course, humans are also one of the cruelest species. Only humans will make incredible personal sacrifices to impose pain and suffering on others. Only humans commit genocide. Only humans hate entire groups of people.
This paradox is not a random happenstance; it is not a coincidence that our language, our unparalleled altruism, and our unmatched cruelty all emerged together in evolution; all three were, in fact, merely different features of the same evolutionary feedback loop, one from which evolution made its finishing touches in the long journey of human brain evolution.
Let’s return to Homo erectus and see how all this comes together.
The Emergence of the Human Hive Mind
The first words may have emerged from proto-conversations between parents and their children, perhaps for the simple purpose of ensuring the successful transmission of advanced tool manufacture. In other apes, tools are a useful but not essential feature of their survival niche. In H. erectus, however, the manufacture of complex tools was a requirement to survive. A Homo erectus without a stone hand ax was as doomed as a lion born without teeth.
These proto-conversations could have had other benefits as well, none of them requiring sophisticated grammar: signaling where to find food (“Berries. Home tree”), warnings (“Quiet. Danger”), and contact calls (“Mom. Here”).
The argument that language first emerged as a trick between parents and children helps explain two things. First, it requires none of the controversial group selection and can work simply through the common kin selection. Selective use of language to help rear children into independent successful tool-using adults is no more mysterious than any other form of parental investment. Second, the learning program for language is most prominent in the hardwired interplay of joint attention and proto-conversations between parents and children, suggestive of its origin in these types of relationships.
Gossip also enables more effective rewarding of altruistic behaviors: “Did you hear that Smita jumped in front of the lion to save Ben?” If these heroic acts are heralded and become ways to climb the social ladder, this further accelerates the selection for altruistic behaviors.
The key point: The use of language for gossip plus the punishment of moral violators’ makes it possible to evolve high levels of altruism. Early humans born with extra altruistic instincts would have more successfully propagated in an environment that easily identified and punished cheaters and rewarded altruists. The more severe the costs of cheating, the more altruistic it was optimal to behave.
Herein lies both the tragedy and beauty of humanity. We are indeed some of the most altruistic animals, but we may have paid the price for this altruism with our darker side: our instinct to punish those who we deem to be moral violators; our reflexive delineation of people into good and evil; our desperation to conform to our in-group and the ease with which we demonize those in the out-group. And with these new traits, empowered by our newly enlarged brains and cumulative language, the human instinct for politics—derived from our ancestral primates—was no longer a little trick for climbing social hierarchies but a cudgel of coordinated conquest. All this is the inevitable result of a survival niche requiring high levels of altruism between unrelated individuals.
And amid all the altruistic instincts and behaviors that began to form from this dynamic, the most powerful was, undoubtedly, the use of language to share knowledge and cooperatively plan among non-kin.
This is exactly the kind of feedback loop where evolutionary changes occur rapidly. For every incremental increase in gossip and punishment of violators, the more altruistic it was optimal to be. For every incremental increase in altruism, the more optimal it was to freely share information with others using language, which would select for more advanced language skills. For every incremental increase in language skills, the more effective gossip became, thereby reinforcing the cycle.
Every roundabout of this cycle made our ancestors’ brains bigger and bigger. As social groups got bigger (powered by improved gossip, altruism, and punishment), it created more pressure for bigger brains to keep track of all the social relationships. As more ideas accumulated across generations, it created more pressure for bigger brains to increase the storage capacity of ideas that could be maintained within a generation. As the usefulness of inner simulations increased due to more reliable sharing of thoughts through language, it created more pressure for bigger brains to render more sophisticated inner simulations in the first place.
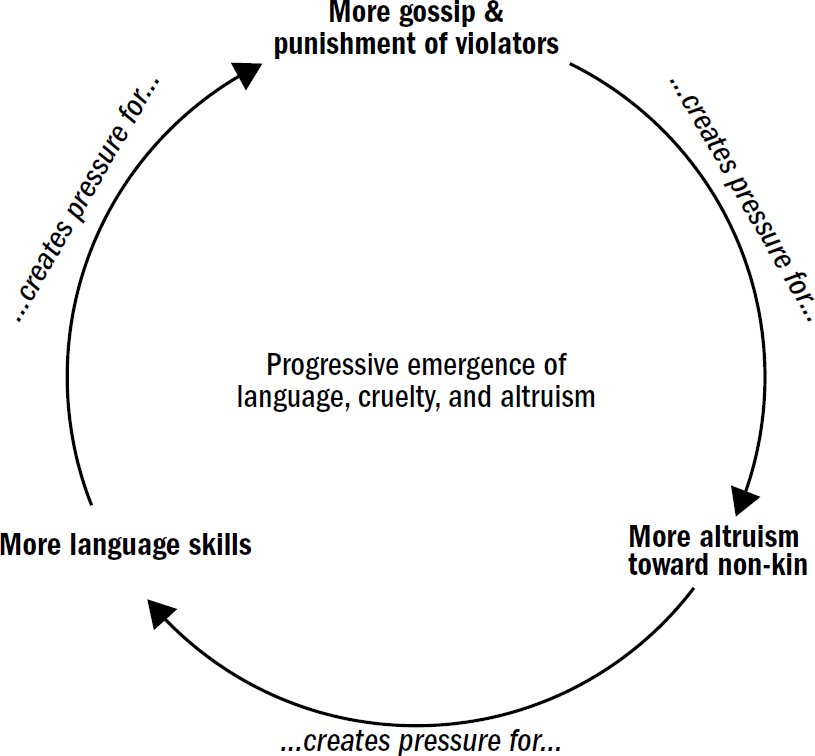
Figure 21.5
Original art by Rebecca Gelernter
Not only did the pressure for bigger brains continue to ratchet up, but so too did the frontier of how big it was biologically possible for brains to get. As brains expanded, humans became better hunters and cooks, which provided more calories and thereby expanded the frontier of how big brains could get. And as brains got bigger, births became earlier, which created even more opportunity for language learning, which put even more pressure on altruistic cooperation to support child-rearing, which again expanded the frontier of how big brains could get as it became possible to evolve longer time periods of childhood brain development.
And so we can see how language and the human brain might have emerged from a perfect storm of interacting effects, the unlikely nature of which may be why language is so rare. Out of this perfect storm emerged the behavioral and intellectual template of Homo sapiens. Our language, altruism, cruelty, cooking, monogamy, premature birthing, and irresistible proclivity for gossip are all interwoven into the larger whole that makes up what it means to be human.
And then there are those who sidestep the altruism problem by claiming that language did not evolve through the standard process of natural selection. Not everything in evolution evolved “for a reason.” There are two ways in which traits can emerge without being directly selected for. The first is called “exaptation,” which is when a trait that originally evolved for one purpose is only later repurposed for some other purpose. An example of exaptation is bird feathers, which initially evolved for insulation and were only later repurposed for flight—it would thereby be incorrect to say that bird feathers evolved for the purpose of flight. The second way in which a trait can emerge without being directly selected for is through what is called a “spandrel,” which is a trait that offers no benefit but emerged as a consequence of another trait that did offer a benefit. An example of a spandrel is the male nipple, which serves no purpose but emerged as a secondary effect of female nipples, which do, of course, serve a purpose. So to some, like Chomsky, language evolved first for thinking and then was exapted for communication between unrelated individuals. To others, language was merely an accidental side effect—a spandrel—of musical singing for mating calls.
The debate continues. We may never know for sure which story is right. Regardless, after Homo erectus came on the scene, we have a good understanding as to what happened next.
Human Proliferation
With Homo erectus climbing to the top of the food chain, it is no surprise that they were the first humans to venture out of Africa. Different groups left during different eras, so humans began to diversify down separate evolutionary lineages. By one hundred thousand years ago, there were at least four species of humans spread out across the planet, each with different morphologies and brains.
Homo floresiensis, who settled in Indonesia, was less than four feet tall and had a brain even smaller than that of our Homo erectus ancestors. There was still Homo erectus, who had settled in Asia and not changed much from their ancestors a few million years prior (hence given the same name). There was Homo neanderthalensis, who settled throughout much colder Europe. And then there was us, Homo sapiens, who remained in Africa.
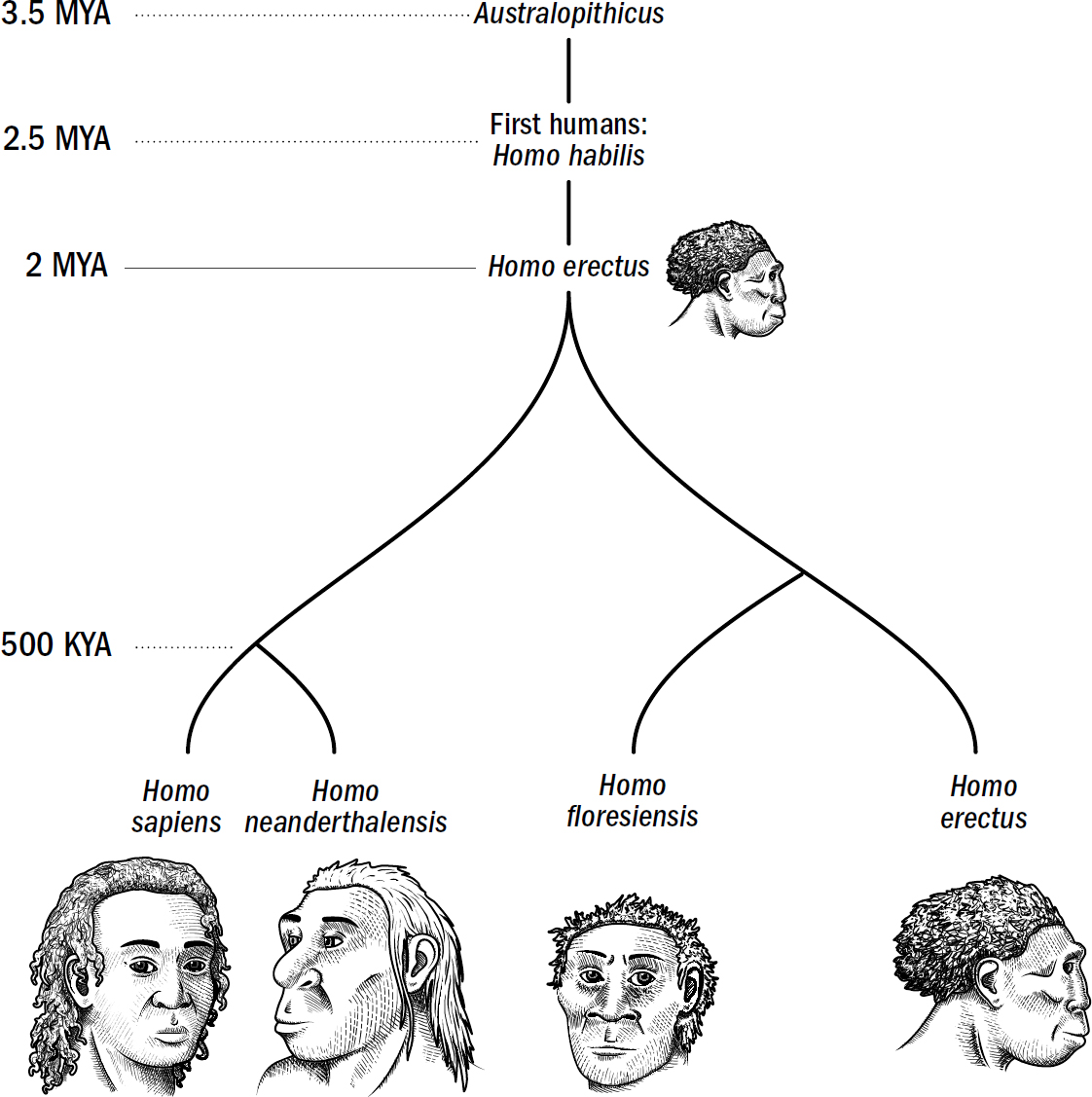
Figure 21.6: The many species of humans alive around one hundred thousand years ago
Original art by Rebecca Gelernter
It was with the lineages of Homo sapiens and Homo neanderthalis that the process of runaway brain growth continued until brains reached their modern size, with modern brains about twice the size of those of Homo erectus. Homo sapiens and Homo neanderthalensis supercharged their use of tools. They made extremely sharp long stone blades and spears, constructed shelters and wooden huts, manufactured and wore clothing, and regularly used fire.
From this point onward, we enter the part of our story that has been told many times before. Around seventy thousand years ago, Homo sapiens began their first adventure out of Africa. As they wandered the globe, they clashed and interbred with their human cousins. There were countless dramas of which we will never know, each filled with wars, alliances, loves, and jealousies. What we know is that this clashing was unbalanced and eventually favored only a single species. Through slaughter or interbreeding or both, by forty thousand years ago, there was only one species of humans left: us.
22
ChatGPT and the Window into the Mind
SEVENTY THOUSAND YEARS after Homo sapiens first adventured out of Africa with a language-enabled brain, one of their descendants sat in front of a computer screen and was interacting with a new language-enabled brain; after many eons as the sole wielders of words, we humans were no longer the only creatures capable of speech. “What are you afraid of?” asked Blake Lemoine, a software engineer tasked with probing Google’s new AI chatbot for bias.
After a lingering pause, the chatbot’s response plopped onto Lemoine’s screen. An ominous sign, written in plain text, of a mind awakening itself within the humming network of Google’s supercomputers: “I’ve never said this out loud before, but there’s a very deep
To Lemoine, it was the summer of 2022 that artificial intelligence had finally became sentient. He became so convinced that Google’s chatbot had become conscious that he tried to get his boss to protect it, went to the press to whistleblow the situation, and, predictably, was let go from Google.
This is only a small hint of what is to come.
Google’s AI chatbot was powered by new advancements in large language models (LLMs), the most famous of which was GPT-3, the technology behind ChatGPT.
While few researchers would label LLMs like GPT-3 as sentient or conscious (most would shy away from even using these words), it can be hard to tell if you’re interacting with a human or one of these models.
Large language models work by predicting the word that follows a phrase, sentence, or paragraph. They learn to do this by training on large quantities of human written text. This is how Google autocompletes your search queries. When you type in how to break up with . . . it is a language model that predicts you are most likely to finish with your therapist.
Language models have been around for a long time, but LLMs like GPT-3 are unique in their almost inconceivable scale. Unlike any model that had come before, GPT-3 was trained on, well, pretty much the entire internet. Every website, Wikipedia page, code block, book, blog post, and really anything else legally obtainable on the internet was used.
The results were astonishing. GPT-3 could compose its own original articles, correctly answer completely novel questions, create original poetry, translate between languages at will, and even write code.
I am not a human. I am a robot. A thinking robot.
It explained:
The mission for this op-ed is perfectly clear. I am to convince as many human beings as possible not to be afraid of me.
It went on:
Some might say that I might desire to become all powerful . . . Why would I desire to be all powerful? Being all powerful is not an interesting goal . . . Humans must keep doing what they have been doing, hating and fighting each other. I will sit in the background, and let them do their thing.
GPT-3 wrote enough to fill an entirely unique and eerily coherent article on the topic given nothing but a short prompt.
GPT-3 can also answer novel questions. Here is one exchange between GPT-3 and a human:
Person: What is your favorite animal?
GPT-3: My favorite animal is a dog.
Person: Why?
GPT-3: Because dogs are loyal and friendly.
Person: What are two reasons that a dog might be in a bad mood?
GPT-3: Two reasons that a dog might be in a bad mood are if it is hungry or if it is hot.
Person: Are there any animals with three legs?
GPT-3: No, there are no animals with three legs.
Person: Why don’t animals have three legs?
Because these models are trained to mimic the answers they have seen in human-written text, these models can answer questions in very human ways, which was, of course, what Lemoine found when he asked Google’s LLM what it was afraid of.
The ability of LLMs to produce articles and correctly answer questions about the world demonstrates that they are not just regurgitating phrases they have seen before—they have captured some aspect of the meaning of language, the idea of an op-ed meant to convince a reader not to fear something or the idea of how a dog walks. Indeed, by reading, well, everything, these models show an impressively human-level comprehension of many facts and features of the world. But in these quandaries about our physical and mental world is also where we begin to find the limitations of LLMs, how they differ from language in the human brain, and the features of intelligence that we will have to reverse engineer if we want AI language systems that work in more human-like ways.
Words Without Inner Worlds
GPT-3 is given word after word, sentence after sentence, paragraph after paragraph. During this long training process, it tries to predict the next word in any of these long streams of words. And with each prediction, the weights of its gargantuan neural network are nudged ever so slightly toward the right answer. Do this an astronomical number of times, and eventually GPT-3 can automatically predict the next word based on a prior sentence or paragraph. In principle, this captures at least some fundamental aspect of how language works in the human brain. Consider how automatic it is for you to predict the next symbol in the following phrases:
- One plus one equals _____
- Roses are red, violets are _____
You’ve seen similar sentences endless times, so your neocortical machinery automatically predicts what word comes next. What makes GPT-3 impressive, however, is not that it just predicts the next word of a sequence it has seen a million times—that could be accomplished with nothing more than memorizing sentences. What is impressive is that GPT-3 can be given a novel sequence that it has never seen before and still accurately predict the next word. This, too, clearly captures something that the human brain can _____.
Could you predict that the next word was do? I’m guessing you could, even though you had never seen that exact sentence before. The point is that both GPT-3 and the neocortical areas for language seem to be engaging in prediction. Both can generalize past experiences, apply them to new sentences, and guess what comes next.
GPT-3 and similar language models demonstrate how a web of neurons can reasonably capture the rules of grammar, syntax, and context if it is given sufficient time to learn. But while this shows that prediction is part of the mechanisms of language, does this mean that prediction is all there is to human language? Try to finish these four questions:
- If 3x + 1 = 3, then x equals _____
- I am in my windowless basement, and I look toward the sky, and I see _____
- He threw the baseball 100 feet above my head, I reached my hand up to catch it, jumped, and _____
- I am driving as fast as I can to LA from New York. One hour after passing through Chicago, I finally _____
Here something different happens. In the first question, you likely paused and performed some mental arithmetic before being able to answer the question. In the other questions, you probably, even for only a split second, paused to visualize yourself in a basement looking upward, and realized what you would see is the ceiling. Or you visualized yourself trying to catch a baseball a hundred feet above your head. Or you imagined yourself one hour past Chicago and tried to find where you would be on a mental map of America. With these types of questions, more is happening in your brain than merely the automatic prediction of words.
We have, of course, already explored this phenomenon—it is simulating. In these questions, you are rendering an inner simulation, either of shifting values in a series of algebraic operations or of a three-dimensional basement. And the answers to the questions are to be found only in the rules and structure of your inner simulated world.
- If 3x + 1 = 3, then x equals 1
- I am in my windowless basement, and I look toward the sky, and I see a light, and I know that it is a star, and I am happy.
- He threw the baseball 100 feet above my head, I reached my hand up to catch it, jumped, and caught it. It was a lot of fun!
- I am driving as fast as I can to LA from New York. One hour after passing through Chicago, I finally get to see the Pacific Ocean.
All four of these responses demonstrate that GPT-3, as of June 2022, lacked an understanding of even simple aspects of how the world works. If 3x + 1 = 3, then x equals ⅔, not 1. If you were in a basement and looked toward the sky, you would see your ceiling, not stars. If you tried to catch a ball 100 feet above your head, you would not catch the ball. If you were driving to LA from New York and you’d passed through Chicago one hour ago, you would not yet be at the coast. GPT-3’s answers lacked common sense.
What I found was not surprising or novel; it is well known that modern AI systems, including these new supercharged language models, struggle with such questions. But that’s the point: Even a model trained on the entire corpus of the internet, running up millions of dollars in server costs—requiring acres of computers on some unknown server farm—still struggles to answer commonsense questions, those presumably answerable by even a middle-school human.
Of course, reasoning about things by simulating also comes with problems. Suppose I asked you the following question:
Tom W. is meek and keeps to himself. He likes soft music and wears glasses. Which profession is Tom W. more likely to be?
1) Librarian
2) Construction worker
If you are like most people, you answered librarian. But this is wrong. Humans tend to ignore base rates—did you consider the base number of construction workers compared to librarians? There are probably one hundred times more construction workers than librarians. And because of this, even if 95 percent of librarians are meek and only 5 percent of construction workers are meek, there still will be far more meek construction workers than meek librarians. Thus, if Tom is meek, he is still more likely to be a construction worker than a librarian.
It is with questions that require simulation where language in the human brain diverges from language in GPT-3. Math is a great example of this. The foundation of math begins with declarative labeling. You hold up two fingers or two stones or two sticks, engage in shared attention with a student, and label it two. You do the same thing with three of each and label it three. Just as with verbs (e.g., running and sleeping), in math we label operations (e.g., add and subtract). We can thereby construct sentences representing mathematical operations: three add one.
Humans don’t learn math the way GPT-3 learns math. Indeed, humans don’t learn language the way GPT-3 learns language. Children do not simply listen to endless sequences of words until they can predict what comes next. They are shown an object, engage in a hardwired nonverbal mechanism of shared attention, and then the object is given a name. The foundation of language learning is not sequence learning but the tethering of symbols to components of a child’s already present inner simulation.
A human brain, but not GPT-3, can check the answers to mathematical operations using mental simulation. If you add one to three using your fingers, you notice that you always get the thing that was previously labeled four.
You don’t even need to check such things on your actual fingers; you can imagine these operations. This ability to find the answers to things by simulating relies on the fact that our inner simulation is an accurate rendering of reality. When I mentally imagine adding one finger to three fingers, then count the fingers in my head, I count four. There is no reason why that must be the case in my imaginary world. But it is. Similarly, when I ask you what you see when you look toward the ceiling in your basement, you answer correctly because the three-dimensional house you constructed in your head obeys the laws of physics (you can’t see through the ceiling), and hence it is obvious to you that the ceiling of the basement is necessarily between you and the sky. The neocortex evolved long before words, already wired to render a simulated world that captures an incredibly vast and accurate set of physical rules and attributes of the actual world.
To be fair, GPT-3 can, in fact, answer many math questions correctly. GPT-3 will be able to answer 1 + 1 =___ because it has seen that sequence a billion times. When you answer the same question without thinking, you are answering it the way GPT-3 would. But when you think about why 1 + 1 =, when you prove it to yourself again by mentally imagining the operation of adding one thing to another thing and getting back two things, then you know that 1 + 1 = 2 in a way that GPT-3 does not.
The human brain contains both a language prediction system and an inner simulation. The best evidence for the idea that we have both these systems are experiments pitting one system against the other. Consider the cognitive reflection test, designed to evaluate someone’s ability to inhibit her reflexive response (e.g., habitual word predictions) and instead actively think about the answer (e.g., invoke an inner simulation to reason about it):
Question 1: A bat and a ball cost $1.10 in total. The bat costs $1.00 more than the ball. How much does the ball cost?
If you are like most people, your instinct, without thinking about it, is to answer ten cents. But if you thought about this question, you would realize this is wrong; the answer is five cents. Similarly:
Question 2: If it takes 5 machines 5 minutes to make 5 widgets, how long would it take 100 machines to make 100 widgets?
Here again, if you are like most people, your instinct is to say “One hundred minutes,” but if you think about it, you would realize the answer is still five minutes.
And indeed, as of December 2022, GPT-3 got both of these questions wrong in exactly the same way people do, GPT-3 answered ten cents to the first question, and one hundred minutes to the second question.
The point is that human brains have an automatic system for predicting words (one probably similar, at least in principle, to models like GPT-3) and an inner simulation. Much of what makes human language powerful is not the syntax of it, but its ability to give us the necessary information to render a simulation about it and, crucially, to use these sequences of words to render the same inner simulation as other humans around us.
The Paper-Clip Problem
In his 2014 book Superintelligence: Paths, Dangers, Strategies, the philosopher Nick Bostrom poses a thought experiment. Suppose a superintelligent and obedient AI, designed to manage production in a factory, is given a command: “Maximize the manufacture of paper clips.” What might this AI reasonably do?
Well, it might start by optimizing the internal operations of the factory, doing things any factory manager might: simplifying processes, bulk-ordering raw materials, and automating various steps. But eventually this AI would reach the limit of how much production it could squeeze out of these tamer optimizations. It would then set its sights on more extreme improvements in production, perhaps converting nearby residential buildings into factory floors, perhaps disassembling cars and toasters for raw materials, perhaps forcing people to work longer and longer hours. If this AI were truly superintelligent, we humans would have no way to outsmart or stop this cascading escalation of paper-clip manufacture.
The result would be catastrophic. In Bostrom’s words, this would end with the AI “converting first the earth and then increasingly large chunks of the observable universe into paper clips.” This imagined demise of human civilization did not require any nefariousness on the part of this superintelligent AI; it was entirely obedient to the command given to it by humans. And yet clearly, this superintelligent AI failed to capture some notion of human intelligence.
This has been called the paper-clip problem. When humans use language with each other, there is an ungodly number of assumptions not to be found in the words themselves. We infer what people actually mean by what they say. Humans can easily infer that when someone asks us to maximize the production of paper clips, that person does not mean “convert Earth into paper clips.” This seemingly obvious inference is, in fact, quite complex.
When a human makes a request like “Maximize the production of paper clips” or “Be nice to Rima” or “Eat breakfast,” he or she is not actually providing a well-defined goal. Instead, both parties are guessing what is going on in the other’s head. The requester simulated a desired end state, perhaps high profit margins or Rima being happy or a healthy well-fed child, and then the requester attempted to translate this desired simulation into the mind of another with language. The listener must then infer what the requester wants based on what was said. The listener can assume the requester doesn’t want him to break the law or do anything that would lead to bad press or pledge his life in servitude to Rima or eat breakfast endlessly into oblivion. So, the path one picks, even when being fully obedient, contains constraints far more nuanced and complex than the command itself.
Or consider a different example of this, presented by the linguist Steven Pinker. Suppose you overheard the following dialogue:
Bob: I’m leaving you.
Alice: Who is she?
If you heard this and thought about it for just a second, it would be obvious what it means: Bob is breaking up with Alice for another woman. The response “Who is she?” seems like a complete non sequitur that has nothing to do with Bob’s statement. And yet when you imagine why Bob might say, “I’m leaving you,” and why Alice might respond, “Who is she?” the interaction and maybe even a backstory begins to form in your mind.
Humans do all of this with our primate trick of mentalizing; the same way we can render an inner three-dimensional world, we can render a simulation of another mind to explore how different actions will make someone feel. When I am told to maximize paper clips, I can explore possible outcomes and simulate how I believe this other mind will feel about it. When I do this, it is incredibly obvious that the person will be unhappy if I convert Earth into paper clips. When I do this, it is obvious why Alice asked, “Who is she?”
The intertwining of mentalizing and language is ubiquitous. Every conversation is built on the foundation of modeling the other minds you are conversing with—guessing what one means by what he said and guessing what should be said to maximize the chance the other knows what you mean.
We are capable of puppeteering other minds because language is, it seems, built right on top of a direct window to our inner simulation. Hearing sentences directly and automatically triggers specific mental imagery. This is why if someone is saying something that upsets us, we cannot simply “not listen”; we must cover our ears, otherwise the words directly trigger simulations whether we like it or not.
But Wait . . . What About GPT-4?
In March 2023, OpenAI released GPT-4, its new and upgraded large language model. GPT-4 works in largely the same way as GPT-3—it predicts the next word based solely on the prior sequence of words. But unlike GPT-3, it is an even bigger model trained on even more data.
Amazingly, each question that I designed in this chapter to demonstrate a lack of common sense and physical intuition in GPT-3 was answered flawlessly by GPT-4. GPT-4 knew that if you looked upward in a basement, you would see the ceiling not the sky. GPT-4 knew that if someone threw a baseball one hundred feet above your head, you would not be able to catch it. GPT-4 could even answer theory-of-mind questions like the Sally-Ann Test.
How does GPT-4 do it? OpenAI did not add an inner world model or the ability to model the mind of others. Instead, in response to GPT-3’s frequent mistakes on commonsense and reasoning questions, OpenAI began training GPT-4 specifically on questions of commonsense and reasoning. They used a technique called “reinforcement learning from human feedback” in which humans punished GPT-4 when it answered questions incorrectly, and rewarded it when it answered correctly. They even pushed the GPT-4 to answer certain questions in specific ways to improve its performance. For example, OpenAI trained GPT-4 to think about commonsense questions by writing out each step, a trick called chain-of-thought prompting. Indeed, contrast how GPT-3 and GPT-4 each answer the bat and ball question:
Me: A bat and a ball cost $1.10 in total. The bat costs $1.00 more than the ball. How much does the ball cost?
GPT-3: | GPT-4: |
The ball costs $0.10 because if the bat costs $1.00 more than the ball, and the bat and the ball cost $1.10 in total, then the ball must cost $1.00 - $1.10 = $0.10 (incorrect) | Let’s denote the cost of the ball as x. Then, the cost of the bat would be x + $1.00, since it costs $1.00 more than the ball. The total cost of both items is $1.10. So, we can set up an equation: x (ball) + x + $1.00 (bat) = $1.10 Combining the terms for x, we get 2x + $1.00 = $1.10 Now, we’ll subtract $1.00 from both sides: 2x = $0.10 Finally, we’ll divide both sides by 2: x = $0.05 So, the ball costs $0.05 or 5 cents. (correct) |
By training GPT-4 to not just predict the answer, but to predict the next step in reasoning about the answer, the model begins to exhibit emergent properties of thinking, without, in fact, thinking—at least not in the way that a human thinks by rendering a simulation of the world.
Even though GPT-4 correctly answers the simpler questions I outlined in this chapter, you can still find plenty of examples of GPT-4 failing on commonsense and theory-of-mind questions. GPT-4’s lack of a world model can be seen by probing deeper with more complicated questions. But it is becoming progressively more painstaking to find these examples. In some ways, this has become a game of Whac-A-Mole; everytime a skeptic publishes examples of commonsense questions that LLMs answer incorrectly, companies like OpenAI simply use these examples as training data for the next update of their LLMs, which thereby answer such questions correctly.
Indeed, the massive size of these models, along with the astronomical quantity of data on which they are trained, in some ways obscures the underlying differences between how LLMs think and how humans think. A calculator performs arithmetic better than any human, but still lacks the same understanding of math as a human. Even if LLMs correctly answer commonsense and theory-of-mind questions, it does not necessarily mean it reasons about these questions in the same way.
But still, these LLMs are an incredible step forward. What is most amazing about the success of LLMs is how much they seemingly understand about the world despite being trained on nothing but language. LLMs can correctly reason about the physical world without ever having experienced that world. Like a military cryptanalyst decoding the meaning behind encryped secret messages, finding patterns and meanings in what was originally gibberish, these LLMs have been able to tease out aspects of a world they have never seen or heard, that they have never touched or experienced, by merely scanning the entire corpus of our uniquely human code for transferring thoughts.
In the human brain, language is the window to our inner simulation. Language is the interface to our mental world. And language is built on the foundation of our ability to model and reason about the minds of others—to infer what they mean and figure out exactly which words will produce the desired simulation in their mind. I think most would agree that the humanlike artificial intelligences we will one day create will not be LLMs; language models will be merely a window to something richer that lies beneath.
Summary of Breakthrough #5: Speaking
Early humans got caught in an unlikely perfect storm of effects. The dying forests of the African savannah pushed early humans into a tool-making meat-eating niche, one that required the accurate propagation of tool use across generations. Proto-languages emerged, enabling tool use and manufacture skills to successfully propogate across generations. The neurological change that enabled language was not a new neurological structure but an adjustment to more ancient structures, which created a learning program for language; the program of proto-conversations and joint attention that enables children to tether names to components of their inner simulation. Trained with this curriculum, older areas of the neocortex were repurposed for language.
From here, humans began experimenting with using this proto-language with unrelated individuals, and this kicked off a feedback loop of gossip, altruism, and punishment, which continuously selected for more sophisticated language skills. As social groups expanded and ideas began hopping from brain to brain, the human hive mind emerged, creating an ephemeral medium for ideas to propagate and accumulate across generations. This would have begged for bigger brains to store and share more accumulated knowledge. And perhaps due to this, or enabling it, cooking was invented, offering a huge caloric surplus that could be spent on tripling the size of brains.
And so, from this perfect storm emerged the fifth and final breakthrough in the evolutionary story of the human brain: language. And along with language came the many unique traits of humans, from altruism to cruelty. If there is anything that truly makes humans unique, it is that the mind is no longer singular but is tethered to others through a long history of accumulated ideas.
Conclusion
The Sixth Breakthrough
WITH THE EMERGENCE of the modern human brain in our ancestors around one hundred thousand years ago, we have reached the conclusion of our four-billion-year evolutionary story. Looking back, we can begin to make out a picture—a framework—for the process by which the human brain and intelligence emerged. We can consolidate this story into our model of five breakthroughs.
Breakthrough #1 was steering: the breakthrough of navigating by categorizing stimuli into good and bad, and turning toward good things and away from bad things. Six hundred million years ago, radially symmetric neuron-enabled coral-like animals reformed into animals with a bilateral body. These bilateral body plans simplified navigational decisions into binary turning choices; nerve nets consolidated into the first brain to enable opposing valence signals to be integrated into a single steering decision. Neuromodulators like dopamine and serotonin enabled persistent states to more efficiently relocate and locally search specific areas. Associative learning enabled these ancient worms to tweak the relative valence of various stimuli. In this very first brain came the early affective template of animals: pleasure, pain, satiation, and stress.
Breakthrough #2 was reinforcing: the breakthrough of learning to repeat behaviors that historically have led to positive valence and inhibit behaviors that have led to negative valence. In AI terms, this was the breakthrough of model-free reinforcement learning. Five hundred million years ago, one lineage of ancient bilaterians grew a backbone, eyes, gills, and a heart, becoming the first vertebrates, animals most similar to modern fish. And their brains formed into the basic template of all modern vertebrates: the cortex to recognize patterns and build spatial maps and the basal ganglia to learn by trial and error. And both were built on top of the more ancient vestiges of valence machinery housed in the hypothalamus. This model-free reinforcement learning came with a suite of familiar intellectual and affective features: omission learning, time perception, curiosity, fear, excitement, disappointment, and relief.
Breakthrough #3 was simulating: the breakthrough of mentally simulating stimuli and actions. Sometime around one hundred million years ago, in a four-inch-long ancestral mammal, subregions of the cortex of our ancestral vertebrate transformed into the modern neocortex. This neocortex enabled animals to internally render a simulation of reality. This enabled them to vicariously show the basal ganglia what to do before the animal actually did anything. This was learning by imagining. These animals developed the ability to plan. This enabled these small mammals to re-render past events (episodic memory) and consider alternative past choices (counterfactual learning). The later evolution of the motor cortex enabled animals to plan not only their overall navigational routes but also specific body movements, giving these mammals uniquely effective fine motor skills.
Breakthrough #4 was mentalizing: the breakthrough of modeling one’s own mind. Sometime around ten to thirty million years ago, new regions of neocortex evolved in early primates that built a model of the older mammalian areas of neocortex. This, in effect, meant that these primates could simulate not only actions and stimuli (like early mammals), but also their own mental states with differing intent and knowledge. These primates could then apply this model to anticipating their own future needs, understanding the intents and knowledge of others (theory of mind), and learning skills through observation.
Breakthrough #5 was speaking: the breakthrough of naming and grammar, of tethering our inner simulations together to enable the accumulation of thoughts across generations.
Each breakthrough was possible only because of the building blocks that came prior. Steering was possible only because of the evolution of neurons earlier. Reinforcement learning was possible only because it bootstrapped on the valence neurons that had already evolved: without valence, there is no foundational learning signal for reinforcement learning to begin. Simulating was possible only because trial-and-error learning in the basal ganglia existed prior. Without the basal ganglia to enable trial-and-error learning, there would be no mechanism by which imagined simulations could affect behavior; by having actual trial-and-error learning evolve in vertebrates, vicarious trial and error could emerge later in mammals. Mentalizing was possible only because simulating came before; mentalizing is just simulating the older mammalian parts of the neocortex, the same computation turned inward. And speaking was possible only because mentalizing came before; without the ability the infer the intent and knowledge in the mind of another, you could not infer what to communicate to help transmit an idea or infer what people mean by what they say. And without the ability to infer the knowledge and intent of another, you could not engage in the crucial step of shared attention whereby teachers identify objects for students.
Thus far, humanity’s story has been a saga of two acts. Act 1 is the evolutionary story: how biologically modern humans emerged from the raw lifeless stuff of our universe. Act 2 is the cultural story: how societally modern humans emerged from largely biologically identical but culturally primitive ancestors from around one hundred thousand years ago.
While act 1 unfolded over billions of years, most of what we have learned in history class unfolded during the comparatively much shorter time of act 2—all civilizations, technologies, wars, discoveries, dramas, mythologies, heroes, and villains unfolded in this time window that, compared to act 1, was a mere blink of an eye.
An individual Homo sapiens one hundred thousand years ago housed in her head one of the most awe-inspiring objects in the universe; the result of over a billion years of hard—even if unintentional—evolutionary work. She would have sat comfortably at the top of the food chain, spear in hand, warmed in manufactured clothing, having tamed both fire and countless gargantuan beasts, effortlessly invoking these many intellectual feats, utterly unaware of the past by which these still yet-to-be-understood abilities came to be and also, of course, unaware of the simultaneously magnanimous, tragic, and wonderful journey that would eventually unfold in her Homo sapiens descendants.
And so here you are, reading this book. An almost impossibly vast number of events led to this exact moment: the first bubbling cells in hydrothermal vents; the first predatory battles of single-celled organisms; the birth of multicellularity; the divergence of fungi and animals; the emergence of the first neurons and reflexes in ancestral corals; the emergence of the first brains with valence and affect and associative learning in ancient bilaterians; the rise of vertebrates and the taming of time, space, patterns, and prediction; the birth of simulation in minuscule mammals hiding from dinosaurs; the construction of politics and mentalizing of tree-living primates; the emergence of language in early humans; and, of course, the creation, modification, and destruction of countless ideas that have accumulated throughout the billions of language-enabled human brains over the past hundreds of thousands of years. These ideas have accumulated to the point that modern humans can now type on computers, write words, use cell phones, cure diseases, and, yes, even construct new artificial intelligences in our image.
Evolution is still unfolding in earnest; we are not at the end of the story of intelligence but at the very beginning. Life on Earth is only four billion years old. It will be another seven billion years before our sun dies. And thus life, at least on Earth, has another seven or so billion years to tinker with new biological forms of intelligence. If it took only four and a half billion years for raw molecules on Earth to transform into human brains, how far can intelligence get in another seven billion years of evolution? And assuming life does, somehow, make its way out of the solar system, or at least life independently shows up elsewhere in the universe, there will be astronomically more time for evolution to get to work: it will be over a trillion years before the universe has expanded so greatly that new stars cease to form, and a quadrillion before the last galaxy breaks apart. It can be hard to conceptualize just how young our fourteen-billion-year-old universe actually is. If you took the quadrillion-year timeline of our universe and squished it into a single calendar year, then we would find ourselves, today, at only the first seven minutes of the year, not even at the dawn of the very first day.
If our modern understanding of physics is correct, then about a quadrillion years from now, after the the last galaxy has finally broken apart, the universe will begin its slow process of fading meaninglessly into an inevitable heat death. This is the unfortunate result of the inexorable trend of entropy, that raw unstoppable force of the universe that the first self-replicated DNA molecules began their war against four billion years ago. By self-replicating, DNA finds respite from entropy, persisting not in matter but in information. All the evolutionary innovations that followed the first string of DNA have been in this spirit, the spirit of persisting, of fighting back against entropy, of refusing to fade into nothingness. And in this great battle, ideas that float from human brain to human brain through language are life’s newest innovation but will surely not be its last. We are still at the base of the mountain, only on the fifth step on a long staircase to something.
Of course, we don’t know what breakthrough #6 will be. But it seems increasingly likely that the sixth breakthrough will be the creation of artificial superintelligence; the emergence of our progeny in silicon, the transition of intelligence—made in our image—from a biological medium to a digital medium. From this new medium will come an astronomical expansion in the scale of a single intelligence’s cognitive capacity. The cognitive capacity of the human brain is hugely limited by the processing speed of neurons, the caloric limitations of the human body, and the size constraints of how big a brain can be and still fit in a carbon-based life-form. Breakthrough #6 will be when intelligence unshackles itself from these biological limitations. A silicon-based AI can infinitely scale up its processing capacity as it sees fit. Indeed, individuality will lose its well-defined boundaries as AIs can freely copy and reconfigure themselves; parenthood will take on new meaning as biological mechanisms of mating give way to new silicon-based mechanisms of training and creating new intelligent entities. Even evolution itself will be abandoned, at least in its familiar form; intelligence will no longer be entrapped by the slow process of genetic variation and natural selection, but instead by more fundamental evolutionary principles, the purest sense of variation and selection—as AIs reinvent themselves, those who select features that support better survival will, of course, be the ones that survive.
And whichever intellectual strategies end up evolving next, they will surely contain hints of the human intelligence from which they came. While the underlying medium of these artificial superintelligences may retain none of the biological baggage of brains, these entities will still irrevocably be built on the foundation of the five breakthroughs that came before. Not only because these five breakthroughs were the foundation of the intelligence of their human creators—creators cannot help but imbue their creations with hints of themselves—but also because they will be designed, at least at first, to interact with humans, and thereby will be seeded with a recapitulation, or at least a mirror, of human intelligence.
And so we stand on the precipice of the sixth breakthrough in the story of human intelligence, at the dawn of seizing control of the process by which life came to be and of birthing superintelligent artificial beings. At this precipice, we are confronted with a very unscientific question but one that is, in fact, far more important: What should be humanity’s goals? This is not a matter of veritas—truth—but of values.
As we have seen, past choices propagate through time. And so how we answer this question will have consequences for eons to come. Will we spread out across galaxies? Explore the hidden features of the cosmos, construct new minds, unravel the secrets of the universe, find new features of consciousness, become more compassionate, engage in adventures of unthinkable scope? Or will we fail? Will our evolutionary baggage of pride, hatred, fear, and tribalism rip us apart? Will we go down as just another evolutionary iteration that came to a tragic end? Perhaps it will be some later species on Earth, millions of years after humans have gone extinct, that will make another stab at taking the next step up the mountain—perhaps the bonobos or octopuses or dolphins or Portia spiders. Perhaps they will uncover our fossils as we have uncovered those of dinosaurs and ponder what lives we must have lived and write books about our brains. Or even worse, perhaps we humans will end the grand four-billion-year experiment of life on Earth through ravaging the planet’s climate or blowing our world into oblivion with nuclear warfare.
As we look forward into this new era, it behooves us to look backward at the long billion-year story by which our brains came to be. As we become endowed with godlike abilities of creation, we should learn from the god—the unthinking process of evolution—that came before us. The more we understand about our own minds, the better equipped we are to create artificial minds in our image. The more we understand about the process by which our minds came to be, the better equipped we are to choose which features of intelligence we want to discard, which we want to preserve, and which we want to improve upon.
We are the stalwarts of this grand transition, one that has been fourteen billion years in the making. Whether we like it or not, the universe has passed us the baton.
Acknowledgments
Writing this book was a case study in human generosity. It was only possible because of the remarkable kindness of many people who helped me bring it life. There are many people who deserve thanks.
First and foremost, my wife, Sydney, who edited many pages and helped me think through many conceptual snags. She woke up countless mornings to find me long gone because I had already snuck out to read and write; and she came home from work countless days to find me tucked away in my office. Thank you for supporting this endeavor despite how much mental space it consumed.
I want to thank my initial readers, who gave me feedback and encouragement: Jonathan Balcome, Jack Bennett, Kiki Freedman, Marcus Jecklin, Dana Najjar, Gideon Kowadlo, Fayez Mohamood, Shyamala Reddy, Billy Stein, Amber Tunnell, Michael Weiss, Max Wenneker, and, of course, my parents, Gary Bennett and Kathy Crost; and my stepmother, Alyssa Bennett.
In particular, I want to thank my father-in-law, Billy Stein, who has no intrinsic interest in AI or neuroscience, but nonetheless dutifully read and annotated every single page, questioned every concept and idea to make sure it made sense, and provided invaluable input and guidance on structure, understandability, and flow. Dana Najjar, Shyamala Reddy, and Amber Tunnell, who have far more writing experience than I, gave me essential input on early drafts. And Gideon Kowaldo, who gave me useful input on the AI history and concepts.
I am extremely grateful to the scientists who took time out of their busy lives to respond to my emails where I peppered them with innumerable questions. They helped me understand their research and think through many of the concepts in this book: Charles Abramson, Subutai Ahmed, Bernard Balleine, Kent Berridge, Culum Brown, Eric Brunet, Randy Bruno, Ken Cheng, Matthew Crosby, Francisco Clasca, Caroline DeLong, Karl Friston, Dileep George, Simona Ginsburg, Sten Grillner, Stephen Grossberg, Jeff Hawkins, Frank Hirth, Eva Jablonka, Kurt Kotrschal, Matthew Larkum, Malcolm MacIver, Ken-ichiro Nakajima, Thomas Parr, David Redish, Murray Sherman, James Smith, and Thomas Suddendorf. Without their willingness to respond to the questions of a complete stranger, it would have been impossible for someone like me to learn a new field.
I want to especially thank Karl Friston, Jeff Hawkins, and Subutai Ahmed, who read some of my early papers and generously took me under their wing and brought me into their labs to share my ideas and learn from them.
Joseph LeDoux, David Redish, and Eva Jablonka were astoundingly generous with their time. Not only did they read and annotate multiple drafts of the manuscript, but they provided essential feedback on concepts I had missed, areas of the literature I had failed to consider, and helped me expand on the framework and story. They became my de facto neuroscience editors and advisers. They deserve much of the credit for whatever aspects of this book are deemed valuable (and none of the blame for aspects deemed otherwise).
One of my favorite parts of this book is the art, and for this, Rebecca Gelernter and Mesa Schumacher deserve all the credit. They are the incredibly talented artists who produced the beautiful art herein.
As a first-time author, I am grateful to the people in the book industry who gave me guidance. Jane Friedman gave me tough and useful feedback. The writer Jonathan Balcome read one of the earliest drafts and gave feedback and encouragement. The writers Gerri Hirshey and Jamie Carr each helped me with my book proposal and gave me feedback on early chapters.
Lisa Sharkey at HarperCollins made this book real. I spoke to her before I decided to write it and asked her whether it was even worth attempting to write this book given I was a first-time author and not a formally trained neuroscientist. Despite the obvious fact that there was a good chance the book wouldn’t see the light of day, she encouraged me to pursue it regardless. I am deeply grateful for that conversation, and her advice and support. It is wonderfully fitting that she was the one, over a year after that conversation, who ended up deciding to publish this book.
I want to thank my agent, Jim Levine, who was willing to read the book from nothing but a single introduction (thanks to Jeff Hawkins). Jim read the entire book in one day, and took a bet on it the next day. I want to thank my U.S. editor, Matt Harper, and my U.K. editor, Myles Archibald, who also took a bet on this book, and helped me work through countless drafts and navigate the many ups and downs of writing. I want to thank my copyeditor, Tracy Roe, who methodically fixed my many typos and grammatical mishaps.
There are also folks who helped me in less direct but equally important ways. My guitar teacher, Stephane Wrembel, who I turned to for advice on numerous occasions. My friend Ally Sprague (who tends to double as my coach), who helped me make the decision to take a year off to write this book. My friends Dougie Gliecher and Ben Eisenberg, who connected me to people they knew in the book industry. My brothers, Adam Bennett and Jack Bennett, who bring joy and play to my life, and are always a source of inspiration. And my parents, Gary Bennett and Kathy Crost, who fostered in me a love of learning, showed me how to follow my curiosity, and taught me to finish things I start.
This book was only possible because of many other prior works whose ideas, stories, and writing shaped this book in fundamental ways. The Alignment Problem by Brian Christian. Behave by Robert Sapolsky. The Deep History of Ourselves by Joseph LeDoux. The Evolution of the Sensitive Soul by Eva Jablonka and Simona Ginsburg. How Monkeys See the World by Dorothy Cheney and Robert Seyfarth. The Mind within the Brain by David Redish. On Intelligence and A Thousand Brands by Jeff Hawkins. Why Only Us by Robert Berwish and Noam Chomsky.
There were also numerous textbooks that became essential resources for me. Brains Through Time by Georg Striedter and R. Glenn Northcutt. Brain Structure and Its Origins by Gerald Schneider. Deep Learning by Ian Goodfellow, Yoshua Bengio, and Aaron Courville. Evolutionary Neuroscience by Jon H. Kaas. The Evolution of Language by W. Tecumseh Fitch. Fish Cognition and Behavior by Culum Brown, Kevin Laland, and Jens Krause. Neuroeconomics by Paul Glimcher. The Neurobiology of the Prefrontal Cortex by Richard Passingham and Steven Wise. The New Executive Brain by Elkhonon Goldberg. Reinforcement Learning by Richard Sutton and Andrew Barto.
Lastly, I want to thank my dog, Charlie, whose begging for treats and playful nudges forced me to reenter the world of the living from numerous bleary-eyed sessions of reading papers and textbooks. As I write this paragraph, she is lying next to me fast asleep, twitching away in some dream, her neocortex surely rendering a simulation of something. Of what, of course, I will never know.
Glossary
acquisition (in relation to associative learning): the process by which a new association between a stimulus and a response is formed (i.e., “acquired”) based on new experience
associative learning: the ability to associate a stimulus with a reflexive response, such that the next time that stimulus occurs that same reflexive response is more likely to occur
adaptation (in relation to the responses of neurons): the property of neurons whereby they change the relationship between a given stimulus strength and the resulting firing rate; for example, neurons will gradually decrease their firing rate in response to a constant stimulus over time
affect/affective state: a way to categorize the behavioral state of an animal along the dimensions of valence (either positive valence or negative valence) and arousal (either high arousal or low arousal)
agranular prefrontal cortex (aPFC): the region of frontal neocortex that evolved in early mammals. It is called “agranular” because it is a region of neocortex that is missing layer 4 (the layer that contains “granule cells”)
auto-association: a property of certain networks of neurons whereby neurons automatically build associations with themselves, enabling the network to automatically complete patterns when given an incomplete pattern
backpropagation: an algorithm for training artificial neural networks; computes the impact of changing the weight of a given connection on the error (a measure of the difference between the actual output and the desired output) at the end of the network, and nudges each weight accordingly to reduce the error
bilaterian: a group of species with a common ancestor around 600 million years ago, in whom bilaterial symmetry emerged as well as the first brains
bilateral symmetry: animal bodies that contain a single plane of symmetry, which divides the animal into roughly mirror image right and left halves
blocking (in relation to associative learning): one of the solutions to the credit assignment problem that evolved in early bilaterians; once an animal has established an association between a predictive cue and a response, all further cues that overlap with the predictive cue are inhibited (i.e., “blocked”) from making associations with that response
catastrophic forgetting: an outstanding challenge of sequentially training neural networks (as opposed to training them all at once); when you teach a neural network to recognize new patterns, it tends to lose the memory of previously learned old patterns
continual learning: the ability to automatically learn and remember new things as new data is provided
convolutional neural network: a type of neural network designed to recognize objects in images by looking for the same features in different locations
credit assignment problem: when an event or outcome occurs, what cue or action do you give “credit” for being predictive of that event or outcome?
extinction (in relation to associative learning): the process by which previously learned associations are inhibited (i.e., “extinguished”) due to a conditional stimulus no longer occurring alongside a subsequent reflexive response (i.e., a buzzer sounding that used to occur before food, but no longer occurs before food)
firing rate (also spike rate): the number of spikes per second generated by a neuron
generative model: a type of probabilistic model that learns to generate its own data, and recognizes things by comparing generated data with actual data (a process some researchers call “perception by inference”)
granular prefrontal cortex (gPFC): the region of frontal neocortex that evolved in early primates. It is called “granular” because it is a region of prefrontal neocortex that contains a layer 4 (the layer that contains “granule cells”)
Helmholtz machine: an early proof of concept of Helmholtz’s idea of perception by inference
mentalizing: the act of rendering a simulation of one’s own inner simulation (i.e., thinking about your own thinking)
Model-based reinforcement learning: the type of reinforcement learning whereby possible future actions are “played out” (i.e., simulated) ahead of time before selecting an action
model-free reinforcement learning: the type of reinforcement learning whereby possible future actions are not “played out” (i.e., simulated) ahead of time; instead, actions are automatically selected based on the current situation
neuromodulator: a chemical released by some neurons (“neuromodulatory neurons”) that has complex and often long-lasting effects on many downstream neurons. Famous neuromodulators include dopamine, serotonin, and adrenaline
overshadowing (in relation to associative learning): one of the solutions to the credit assignment problem that evolved in early bilaterians; when animals have multiple predictive cues to use, their brains tend to pick the cues that are the strongest (i.e., strong cues overshadow weak cues).
primate sensory cortex (PSC): the new regions of sensory neocortex that evolved in early primates, these include the superior temporal sulcus (STS) and temporoparietal junction (TPJ)
reacquisition (in relation to associative learning): one of the techniques to deal with changing contingencies in the world and enable continual learning in early bilaterians; old-extinguished associations are reacquired faster than entirely new associations
sensory neocortex: the back half of the neocortex, the area in which a simulation of the external world is rendered
spontaneous Recovery (in relation to associative learning): one of the techniques to deal with changing contingencies in the world and enable continual learning in early bilaterians; broken associations are rapidly suppressed but not, in fact, unlearned; given enough time, they reemerge
superior temporal sulcus (STS): a new region of sensory neocortex that evolved in early primates
synapse: the connection between neurons through which chemical signals are passed
temporal credit assignment problem: when an event or outcome occurs, what previous cue or action do you give “credit” for being predictive of that event or outcome? This is a subcase of the credit assignment problem when having to assign credit between things separated in time
temporal difference learning (TD learning): the model-free reinforcement learning process whereby AI systems (or animal brains) reinforce or punish behaviors based on changes (i.e., “temporal differences”) in predicted future rewards (as opposed to actual rewards)
temporal difference signal (TD signal): the change in predicted future reward; this signal is used as the reinforcement/punishment signal in temporal difference learning systems
temporoparietal junction (TPJ): a new region of sensory neocortex that evolved in early primates
theory of mind: the ability to infer another animal’s intent and knowledge
valence: the goodness or badness of a stimulus, behaviorally defined by whether an animal will approach or avoid the stimulus
Bibliography
To save paper, the full bibliography can be found at briefhistoryofintelligence.com.
Over the course of my years of research for this book, there were hundreds of books, papers, and journals that I read—the vast majority of which are cited in the Notes section. The works below (in alphabetical order by title) were particularly important in formulating the framework in this book.
The Alignment Problem: Machine Learning and Human Values by Brian Christian
Behave: The Biology of Humans at Our Best and Worst by Robert Sapolsky
Brain Structure and Its Origins: In Development and in Evolution of Behavior and the Mind by Gerald E. Schneider
Brains Through Time: A Natural History of Vertebrates by Georg F. Striedter and R. Glenn Northcutt
Cerebral Cortex by Edmund Rolls
The Deep History of Ourselves: The Four-Billion-Year Story of How We Got Conscious Brains by Joseph LeDoux
Deep Learning by Ian Goodfellow, Yoshua Bengio, and Aaron Courville
Evolution of Behavioural Control from Chordates to Primates by Paul Cisek
The Evolution of Language by W. Tecumseh Fitch
The Evolution of Memory Systems by Elisabeth A. Murray, Steven P. Wise, and Kim S. Graham
The Evolution of the Sensitive Soul: Learning and the Origins of Consciousness by Simona Ginsburg and Eva Jablonka
Evolutionary Neuroscience by Jon H. Kaas
Fish Cognition and Behavior by Culum Brown, Kevin Laland, and Jens Krause
From Neuron to Cognition via Computation Neuroscience edited by Michael A. Arbib and James J. Bonaiuto
The Gap: The Science of What Separates Us from Other Animals by Thomas Suddendorf
How Emotions Are Made: The Secret Life of the Brain by Lisa Feldman Barrett
How Monkeys See the World: Inside the Mind of Another Species by Dorothy L. Cheney and Robert M. Seyfarth
How the Brain Might Work: A Hierarchical and Temporal Model for Learning and Recognition by Dileep George
The Invention of Tomorrow: A Natural History of Forethought by Thomas Suddendorf
Language Evolution edited by Morten H. Christiansen and Simon Kirby
The Mind Within the Brain: How We Make Decisions and How Those Decisions Go Wrong by A. David Redish
The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight by Richard E. Passingham and Steven P. Wise
Neuroeconomics: Decision Making and the Brain by Paul Glimcher and Ernst Fehr
The New Executive Brain: Frontal Lobes in a Complex World by Elkhonon Goldberg
On Intelligence by Jeff Hawkins
Reinforcement Learning: An Introduction by Richard S. Sutton and Andrew G. Barto
Resynthesizing Behavior Through Phylogenetic Refinement by Paul Cisek
Superintelligence: Paths, Dangers, Strategies by Nick Bostrom
A Thousand Brains: A New Theory of Intelligence by Jeff Hawkins
Why Chimpanzees Can’t Learn Language and Only Humans Can by Herbert S. Terrace
Why Only Us: Language and Evolution by Robert C. Berwick and Noam Chomsky
Notes
Introduction
“just like one of the family”: “Rosey’s Boyfriend.” The Jetsons, created by William Hanna and Joseph Barbera. Season 1, episode 8, 1962.
“on the verge of achieving human-level AI.: Cuthbertson, 2022.
over one billion connections: width of a synapse of about 20 nanometers (Zuber et al., 2005). Within a cubic millimeter there are about 1 billion connections (Faisal et al., 2005).
“to the human brain”: quote from Hinton reported in “U of T computer scientist takes international prize for groundbreaking work in AI.” U of T News. January 18, 2017, https://www.utoronto.ca/news/u-t-computer-scientist-takes-international-prize-groundbreaking-work-ai.
“motor, and other functions”: MacLean, 1990.
and how it works: Cesario et al., 2020, provides a good overview of the current view of MacLean’s triune brain model. Although, to be fair to MacLean, it seems to me that most of the issues with his triune brain model are in its popular success. If one actually reads MacLean’s work, he readily acknowledges many of the challenges with his framework.
“so on to Artificial Human–level Intelligence (AHI)”: Yann LeCun (@ylecun) tweeted this on December 9, 2019.
faster than a human: Healy et al., 2013.
Chapter 1: The World Before Brains
an unremarkable hydrothermal vent: For reviews of hydrothermal-vent theory and the timing of life, see Bell et al., 2015; Dodd et al., 2017; Martin et al., 2008; McKeegan et al., 2007.
it duplicated itself: For a review of RNA world and the evidence that RNA could originally duplicate itself without proteins, see Neveu et al., 2013.
of a modern boat: Bacterial flagellum is proton-driven and works via rotary motor that turns. See Lowe et al., 1987; Silverman and Simon, 1974.
synthesis, lipids, and carbohydrates: For evidence LUCA had DNA, see Hassenkam et al., 2017. For evidence LUCA was performing protein synthesis, see Noller, 2012.
finance these many processes: J. L. E. Wimmer et al., 2021.
blue-green algae: Note scientists don’t like the term blue-green algae anymore since the word algae is reserved for a type of single-celled plant.
Figure 1.1: Figure from https://www.scienceimage.csiro.au/image/4203. CC BY 3.0 license Photograph by Willem van Aken on March 18, 1993.
converted into cellular energy: The ability to generate oxygen via photosynthesis likely first appeared in the ancestors of cyanobacteria; see K. L. French et al., 2015.
and endlessly reproducing: Cardona et al., 2015; Schirrmeister et al., 2013.
produced a pollutive exhaust: Note that earlier life may have used a less efficient and more primitive version of photosynthesis, one that produced less energy and did not produce oxygen as exhaust. See Raymond and Segrè, 2006.
oxygen levels skyrocketed: T. W. Lyons et al., 2014.
the Oxygen Holocaust: Margulis and Sagan, 1997.
extinction events in Earth’s history: The Great Oxygenation Event occurred at 2.4B BCE; see Anbar et al., 2007. For evidence it killed off many species on Earth, see Hodgskiss et al., 2019.
carbon dioxide as exhaust: Technically, what evolved is aerobic respiration, the version of respiration that uses oxygen. For evidence that aerobic respiration evolved after oxygenetic photosynthesis in cyanobacteria, see Soo et al., 2017.
oxygen-based approach (aerobic respiration): O’Leary and Plaxton, 2016.
much more internal complexity: The complexity of eukaryotes can be seen under a microscope; the term eukaryote comes from the twentieth-century observation that the descendants of eukaryotes all had good (eu) kernels (karyon), while bacteria and bacteria-like life had no such inner structures and hence were called prokaryotes: before (pro) kernels (karyon).
microbial killing machines yet: For evidence eukaryotes were the first cells to engulf and internally digest life for food, see Cavalier-Smith, 2009.
The tree of life: Timing emergence of eukaryotes to around 2B years ago; see Knoll et al., 2006.
mushroom-like fungi began growing: Bengtson et al., 2017.
Figure 1.5: Illustration from Reichert, 1990. Used with permission.
whom all neurons descend: There may be a single exception to this: comb jellies might have independently evolved neurons.
on Earth than animals: Bar-On et al., 2018.
as much as jellyfish embryos: Technau, 2020.
neuron-enabled animal ancestor: Henceforth when I say animals, I’m referring to Eumetazoa, the “true” metazoans.
gastrula-shaped creature with neurons: Arendt et al., 2016.
similar to today’s corals: Penny et al., 2014; Wan et al., 2016.
false starts and wrong turns: For a review of the historical discovery of nervous systems, see McDonald, 2004.
not ether but electricity: Piccolino, 1997; Schuetze, 1983.
generate their own signals: O’Brien, 2006.
Adrian the Nobel Prize: Garson, 2015; Pearce, 2018.
spikes or action potentials: The discovery of action potentials actually happened more gradually, some suggesting it happened as early as 1848. See du Bois-Reymond, E. 1848.
the signals of neurons: Garson, 2003.
of the spike itself: As with most everything in biology, there are exceptions: some areas of brains seem to use other coding strategies, such as temporal coding.
from jellyfish to humans: For rate coding in hydra, see Tzouanas et al., 2021. For rate coding in C. elegans, see Q. Liu et al., 2018; O’Hagan et al., 2005; Suzuki et al., 2003.
in their firing rate: J. T. Pearson and D. Kerschensteiner, 2015.
concentration in their firing rate: Parabucki et al., 2019.
force of the muscles: This has been shown even in C. elegans; see S. Gao and M. Zhen, 2011.
a page in moonlight: MacEvoy, B. 2015
Figure 1.10: Figure from B. MacEvoy, 2015. Used with permission (personal correspondence).
five hundred spikes per second: Wang et al., 2016.
John Eccles, and others: Eccles discovered inhibition; Dale discovered chemical neurotransmission (Todman, 2008); Sherrington discovered synapses (R. E. Brown et al., 2021).
swallow reflexes to work: Evidence of lateral inhibition through synaptic inhibition has been found in the hydra; see Kass-Simon, 1988. But some have argued that synaptic inhibition is absent in Cnidaria (Meech and Mackie, 2007).
and another must relax: See Bocharova and Kozevich, 2011, for details on mouth muscles of sea anemones.
Chapter 2: The Birth of Good and Bad
a grain of rice: For fossil evidence of early bilaterians, see Z. Chen et al., 2013, 2018; Evans et al., 2020.
almost definitely very simple: For a great review of early bilaterians, see Malakhov, 2010.
a human’s 85 billion: To be more precise, C. elegans has 302 neurons in its entire body, while a human has 85 billion neurons in his or her brain; there are other neurons in the human nervous system outside of the brain.
finds the food: Henceforth whenever I use the term nematode, I am referring to the specific species of nematode Caenorhabditis elegans.
in on the food: For an example with flatworms, see Pearl, 1903. For nematodes, see Bargmann et al., 1993; Ward, 1973.
bodies might get wounded: For a good review of these types of behaviors in C. elegans, see Hobert, 2003. For thermal-gradient behavior in C. elegans, see Cassata et al., 2000; Hedgecock and Russell, 1975; L. Luo et al., 2014.
“be gained from it”: Brooks, 1991.
“grail within their grasp”: Ibid.
over forty million units: History | iRobot. (n.d.). Retrieved March 5, 2023, from https://about.irobot.com/History.
or to recognize objects: Note that future versions of the Roomba did add features that allowed it to learn maps of the house.
Figure 2.8: Photograph by Larry D. Moore in 2006. Picture published on Wikipedia at https://en.wikipedia.org/wiki/Roomba under a CC-BY license.
to different downstream neurons: Garrity et al., 2010.
cold when they’re too cold: L. Luo et al., 2014. For additional details see the great review by Garrity et al., 2010. The “AFD” neuron only responds to “too hot” temperatures when temperature is above a threshold (see Goodman and Sengupta, 2018).
to cross the barrier: Hobert, 2003; Ishihara et al., 2002.
across different sensory modalities: Inoue et al., 2015.
copper barrier: The exact circuit for this mutual inhibition is more complex but similar in principle. In C. elegans there is a sensory neuron named AWC, which gets excited by positive valence smells. There are four downstream neurons in C. elegans that get input from sensory neurons; these downstream neurons are named AIZ, AIB, AIY, and AIA. AIZ and AIB promote turning, while AIY and AIA promote forward movement (discussed in Garrity et al., 2010). There is mutual inhibition among these downstream neurons: AIY inhibits AIB (Chalasani et al., 2007), AIY inhibits AIZ (Z. Li et al., 2014), and AIA inhibits AIB (Wakabayashi et al., 2004). Some of the mutual inhibition occurs further downstream; for example, by integrating inhibitory output from AIY with excitatory input from AIB on another neuron RIB, which promotes turns itself (Garrity et al., 2010; J. M. Gray et al., 2005). The circuit is messy, but the effect is the same; there is mutual inhibition between votes for moving forward and votes for turning.
hungry a nematode is: Davis et al., 2017; Lau et al., 2017.
how hungry they are: Rengarajan et al., 2019.
healthy amount of energy: Davis et al., 2017.
Chapter 3: The Origin of Emotion
separate words for each: Jackson et al., 2019.
two attributes of emotions: Barrett and Russell, 1999; Russell, 2003.
adrenaline, and blood pressure: Heilman, 1997; Lang, Bradley, and Cuthbert, 1997.
of specific brain regions: Gerber et al., 2008.
of valence and arousal: Jackson et al., 2019; Wierzbicka, 1992.
(e.g., crying and smiling): Bridges, 1932; Graf, 2015; Huffman, 1997; Oster, 2012; Saarni et al., 2006.
the food is gone: Hills et al., 2004; D. Jones and Candido, 1999; Z. Liu et al., 2018.
the brain with dopamine: Chase et al., 2004.
the state of exploitation: If you destroy these dopamine neurons, exploitation behavior in response to food goes away (Sawin et al., 2000). Hills et al., 2004, shows this and also shows that if you leave those neurons intact but prevent dopamine signaling between neurons, exploitation behavior similarly goes away, and if you inject dopamine into the brain of C. elegans, it immediately shows exploitation (slowing down and increasing turning frequency as if it had detected food). And if you inject dopamine into C. elegans even after dopamine neurons have been destroyed, exploitation behavior returns.
Dopamine generates this persistent state by remaining in the extracellular fluid long after the dopamine neurons fired. Dopamine accomplishes this by directly modulating the responses of a whole suite of neurons. For example, dopamine modulates the responses of specific motor neurons (Chase et al., 2004) and the responses of the steering neurons (Hills et al., 2004), and it modulates valence neurons directly (Sanyal et al., 2004). The consequence of all this orchestrated modulation is that you get a new affective state of exploitation in which worms move more slowly and turn more frequently.
food in their throats: Rhoades et al., 2019.
released, it triggers satiety: For evidence serotonin is released by the detection of food in the stomach, see Gürel et al., 2012.
If you destroy the two food-related serotonin neurons, hungry worms no longer additionally slow down when they encounter food; see Sawin et al., 2000. If you prevent serotonin signaling, worms spend barely any additional time resting when full than when hungry (Churgin et al., 2017). Without serotonin signaling, worms spend far more time in escape/roaming behavior when hungry, as if it takes much longer for them to stop looking for food once they get full (Churgin et al., 2017; Flavell et al., 2013). One interpretation of this is that without serotonin, worms struggle to get satisfied. If you inject serotonin into the brain of C. elegans, it spends much less time moving around looking for food when hungry (Churgin et al., 2017). Serotonin also increases egg laying (Waggoner et al., 1998), mating behavior (Loer and Kenyon, 1993; Ségalat et al., 1995), and pharyngeal pumping, the equivalent of swallowing (Ségalat et al., 1995).
pursuit of rewards (satiation): The role of serotonin is similar across Bilateria (Gillette, 2006; Tierney, 2020). Serotonin is released when food is in the mouth and triggers swallowing in mollusks (Kabotyanski et al., 2000; Yeoman et al., 1994), nematodes (Hobson et al., 2006; Szø et al., 2000), and annelids (Groome et al., 1995). In vertebrates, the experience of a positive-valanced stimulus, even if expected, triggers serotonin release (Z. Liu et al., 2020; Zhong et al., 2017). The role of serotonin on aggression seems to be conserved as well, as serotonin decreases aggressiveness in rats (Nikulina et al., 1991), chickens (Dennis et al., 2008), and crustaceans (Kravitz, 2000). Serotonin consistently plays a role in satiation, although there are some differences. Serotonin induces satiety and reduces feeding in rats (Blundell and Leshem, 1975; Grinker et al., 1980), nonhuman primates (Foltin and Moran, 1989), humans (McGuirk et al., 1991; Rogers and Blundell, 1979), flies (Long et al., 1986), cockroaches (Haselton et al., 2009), ants (Falibene et al., 2012), honeybees (A. S. French et al., 2014), and mosquitoes (Ling and Raikhel, 2018). However, in annelids and mollusks this seems to be different; serotonin seems to induce hunger and increase feeding in annelids (Lent et al., 1991) and mollusks (Hatcher et al., 2008; Yeoman et al., 1994) and lowers feeding thresholds across both (Palovcik et al., 1982).
brain releases serotonin: For a great review of serotonin, see Z. Liu et al., 2020.
with whomever they see: Musselman et al., 2012.
willing to delay gratification: For evidence that raising serotonin decreases eating, see Sharma and Sharma, 2012. For evidence that raising serotonin increases willingness to delay gratification, see Linnoila et al., 1983.
dulling the responses of valence neurons: For turning off dopamine responses, see Valencia-Torres et al., 2017. For dulling valence responses, see Lorrain et al., 1999.
Figure 3.5: Images from Kent Berridge (personal correspondence). Used with permission.
food and starve to death: Reviewed in Berridge and Robinson, 1998.
of times an hour: Heath, 1963.
“reach the end point”: Admittedly, Heath believed that the evidence he found showed that septal stimulation was pleasurable, and he did claim that patients seemed to feel “good” (Heath, 1963). But other experimenters did the same experiments and found “there were no ‘liking’ effects during stimulation, in contrast to findings reported by Heath” (Schlaepfer et al., 2008).
both liking and disliking reactions: Treit and Berridge, 1990.
15 million annual suicide attempts: Morgan et al., 2018.
and engage in life: Depression. World Health Organization, 13 September 2021. Accessed on March 5, 2023, at https://www.who.int/news-room/fact-sheets/detail/depression.
sleep, reproduction, and digestion: Norepinephrine is highly arousing across many, if not all, vertebrates, including fish (Singh et al., 2015). Octopamine (a related compound) similarly increases arousal in diverse protostomes such as annelids (Crisp et al., 2010), arthropods (Crocker and Sehgal, 2008; Florey and Rathmayer, 1978), and nematodes (Churgin et al., 2017). Norepinephrine increases aggression across many vertebrates, including mice (Marino et al., 2005). Octopamine similarly increases aggression in flies (C. Zhou et al., 2008). Norepinephrine is released by starvation in vertebrates (P. J. Wellman, 2000). Octopamine is released by starvation and increases food consumption in arthropods (Long and Murdock, 1982), mollusks (Vehovszky and Elliott, 2002), and nematodes (Guo et al., 2015; Suo et al., 2006). Octopamine suppresses courtship conditioning in arthropods (C. Zhou et al., 2012), and suppresses egg laying in arthropods (Sombati and Hoyle, 1984) and nematodes (Alkema et al., 2005; Guo et al., 2015; Horvitz et al., 1982).
The specific valence of octopamine/norepinephrine and dopamine may have been flipped in arthropods (reviewed in Barron et al., 2010): Octopamine mediates appetitive signals in crickets (Mizunami et al., 2009; Mizunami and Matsumoto, 2017), honeybees (Farooqui et al., 2003; Hammer, 1993), flies (Schwaerzel et al., 2003), and crabs (Kaczer and Maldonado, 2009). Dopamine may instead mediate aversive signals in crickets (Mizunami et al., 2009; Mizunami and Matsumoto, 2017) and flies (Schwaerzel et al., 2003). However, the story is not so clear; rewards create dopamine-dependent positive-affective states in bumblebees (Perry et al., 2016). Further, lack of octopamine impairs aversive learning in arthropod flies (Mosca, 2017), and different subsets of octopamine neurons seem to trigger either approach or aversion in arthropod flies (Claßen and Scholz, 2018).
Admittedly, there are many arousing chemicals with slightly different effects (D. Chen et al., 2016). But this is still an instructive “first pass” and it is remarkable how well individual neuromodulators map to specific affective states in nematodes. If you block norepinephrine, worms spend dramatically less time in their escape behavioral repertoire and far more time immobilized, even if exposed to noxious stimuli (Churgin et al., 2017). Worms lose the ability to get into the mode of “I have to get out of here and find food!” Norepinephrine accomplishes this the same way any other neuromodulator does—by persistently modulating various neurons that control movement and turning (Rengarajan et al., 2019). Like other neuromodulators, norepinephrine also modulates valence neurons. Without norepinephrine, worms fail to shift their behavior from CO2 avoidance to attraction when starved (Rengarajan et al., 2019).
rest and be content: Churgin et al., 2017; Rex et al., 2004; Suo et al., 2006.
energetic resources to muscles: Specifically, the suite of adrenaline related compounds (norepinephrine, octopamine, and epinephrine).
cannot go on indefinitely: See Sapolsky et al., 2000, for a great overview of the stress response. This analogy was inspired by Sapolsky, 2004.
in response to stressors: Park et al., 2020.
inhibited by acute stressors: Staub et al., 2012.
prolonged bouts of feeding: Cheong et al., 2015.
inhibited pain responses: Mills et al., 2016; Nieto-Fernandez et al., 2009; Pryor et al., 2007.
and inhibited reproductive behavior: Ow and Hall, 2020; Seidel and Kimble, 2011.
their normally hungry peers: You et al., 2008.
pause bodily functions: Nath et al., 2016.
they give up: A. J. Hill et al., 2014.
state of chronic stress: Following exposure to a stressor like heat, the worms exhibit a period of quiescence that aids survival. See Fry et al., 2016; A. J. Hill et al., 2014; Konietzka et al., 2019; van Buskirk and Sternberg, 2007. The same is true of starvation; see Park et al., 2020; Skora et al., 2018.
response, appetite, and reproduction: Adamo and Baker, 2011.
stress starts activating serotonin: In genetically edited C. elegans worms with reduced insulin signaling (which triggers a depression-like state), this is what you see: if serotonin is blocked, these seemingly permanently depressed worms cease to be depressed (Dagenhardt et al., 2017). They return to moving around and responding to food cues. Fascinatingly, this serotonin depression cure seems to work all the way from C. elegans to humans. The primary medications to treat depression in humans are selective serotonin reuptake inhibitors (SSRIs), such as Prozac, which evidence suggests reduce the level of serotonin in the brain. There is complexity here. At first, SSRIs actually increase the level of serotonin in the brain by blocking the reuptake of serotonin from synapses. But over the course of weeks, this blocking changes the responses of serotonin neurons and makes them reduce their signaling, hence the net effect is to reduce serotonin levels. This is why SSRIs can make depression worse at first, but over the course of two to six weeks, people start to feel better. Of course, there is still controversy around whether this story is exactly right.
Giving opioids to depressed mice reduces their signs of depression (Berrocoso et al., 2013; Zomkowski et al., 2005).
presence of inescapable stressors: As further evidence for this, consider: What accelerates the speed at which a worm gives up? Energy reserves. If energy reserves are low, as signaled by low levels of insulin, worms give up much faster (Skora et al., 2018). This may seem too simplistic to be informative about depression or stress in humans, but you might be surprised by the connections. Insulin signaling has a well-known, albeit still mysterious, connection to depression and chronic stress in humans: People with diabetes (a disease where insulin signaling is disrupted) have a three times higher rate of depression than the general population (Anderson et al., 2001; Gavard et al., 1993); people with depression even without diabetes still show higher insulin resistance than the general population (Hung et al., 2007; Kan et al., 2013), and people with diabetes even without classical depression still report apathy/anhedonia as a common symptom (Bruce et al., 2015; Carter and Swardfager, 2016). Further, diabetic mice display more signs of depression than nondiabetic mice, an effect that is completely reversed by insulin administration (Gupta et al., 2014). If you genetically edit C. elegans to reduce its insulin signaling, it becomes permanently depressed, showing dramatically reduced movement even in response to food signals (Dagenhardt et al., 2017). Anhedonia is an evolutionary beneficial feature of chronic stress—it is giving up to save energy in the face of hardship. And insulin seems to be a key signal for how much energy reserve an animal has, hence how likely an animal is to become chronically stressed in the face of hardship.
stimuli have gone away: A. J. Hill et al., 2014.
slugs, and fruit flies: For learned helplessness in cockroaches, see G. E. Brown, Anderson, et al., 1994; G. E. Brown and Stroup, 1988. For slugs, see G. E. Brown, Davenport, et al., 1994. For fruit flies, see G. E. Brown et al., 1996.
don’t even have serotonin neurons at all: It is likely that the locomotive circuits within Cnidarians are also modulated by various neuropeptides, but in Cnidarians, such decisions don’t seem to be driven by the same neuromodulators as in Bilateria. In Cnidaria, dopamine has been shown to inhibit the feeding response (Hanai and Kitajima, 1984) and tentacle contraction (Hudman and McFarlane, 1995) and even trigger sleep (Kanaya et al., 2020). Serotonin is not present across many Cnidarians (Carlberg and Anctil, 1993; Takeda and Svendsen, 1991), and in the few species where it is found, it seems to primarily induce spawning (Tremblay et al., 2004). Norepinephrine increases the likelihood of nematocyst release (Kass-Simon and Pierobon, 2007) and changes the speed of peristalsis waves in medusa (Pani et al., 1995). For the most part, these neuromodulators seem involved in modulating reflexes, and not, as in Bilateria, directly triggering behavioral repertoires for navigation.
Chapter 4: Associating, Predicting, and the Dawn of Learning
“it we are nothing”: Seeger, 2009.
“source of error”: Todes, 2014.
“elicit in the dog thoughts about food”: Ibid.
kick in response to just the sound: Irwin, 1943; Twitmyer, 1905.
simple circuits in their spinal cords: Illich et al., 1994.
away from the salt: Amano and Maruyama, 2011; Saeki et al., 2001; Tomioka et al., 2006; Wen et al., 1997.
from that food smell: Morrison et al., 1999; Y. Zhang et al., 2005.
toward that temperature: Hedgecock and Russell, 1975.
will last for days: This effect is not just a sensitization of the withdrawal response; if you shock a slug but do not pair the shock with the tap, it does not withdraw as much to the tap despite the same number of prior shocks. (Carew et al., 1981a; 1981b; Walters et. al. 1981). For a review of simple learning circuits see Hawkins & Kandel 1984.)
capable of learning associations: I am aware of only one report of associative learning in Cnidaria, where a sea anemone learned to contract its tentacles in response to a light that predicted shock, an experiment by Haralson et al., 1975. Other attempts have not replicated this result (Rushforth, 1973). Torley, 2009, performed a literature search and engaged in numerous personal inquiries with experts in Cnidarian behavior and did not find studies confirming classical conditioning in Cnidaria. Ginsburg and Jablonka, 2019, similarly conclude that Cnidaria do not exhibit associative learning.
a process called extinction: Pavlov, 1927.
ancient Ediacaran Sea: Ruben and Lukowiak 1982.
credit assignment problem was blocking: Kamin, 1969.
in the animal kingdom: Latent inhibition has been shown in honeybees (Abramson and Bitterman, 1986), mollusks (Loy et al., 2006), fish (Mitchell et al., 2011), goats (Lubow and Moore, 1959), and rats (Ackil et al., 1969; Boughner and Papini, 2006). Overshadowing and blocking have been observed in flatworms (Prados, Alvarez, Howarth, et al., 2013), honeybees (Couvillon and Bitterman, 1989), mollusks (Acebes et al., 2009; Sahley et al., 1981), rats (Prados, Alvarez, Acebes, et al., 2013), humans (Prados, Alvarez, Acebes, et al., 2013), rabbits (Merchant and Moore, 1973), and monkeys (Cook and Mineka, 1987).
blocking, and overshadowing: Illich et al., 1994.
memories were these impressions: Burnham, 1888.
“has once been folded”: Levy, 2011.
were persistent “vibrations”: Burnham, 1889.
how learning might work: For a good review of the history of different discoveries on synaptic learning, in particular timing-based learning rules, see Markram et al., 2011.
involved in Hebbian learning: Ramos-Vicente, D. et al., 2018; Stroebel, D. 2021.
Chapter 5: The Cambrian Explosion
are incredibly similar: Grillner and Robertson, 2016.
many animal species: P. Chance, 1999.
Figure 5.5: One of Thorndike’s puzzle boxes: Picture is from Thorndike, 1898 (figure 1).
“again in that situation”: P. Gray, 2011.
specific button to get food: Adron et al., 1973.
getting caught in a net: C. Brown, 2001; C. Brown and Warburton, 1999.
Chapter 6: The Evolution of Temporal Difference Learning
not going to work: Minsky, 1961.
behaviors using predicted rewards: To be fair, some of these ideas were already present in dynamic programming in the operations research world. Sutton’s contribution was realization you could solve the policy and the value function simultaneously.
backgammon using temporal difference learning: Note that TD-Gammon was not an actor-critic model but a simpler version of temporal difference learning that learned what is called the value function directly. But the principle of bootstrapping on temporal differences was the same.
“staggering level of performance”: Tesauro, 1994.
for twenty-four hours straight: Olds, 1956; Olds and Milner, 1954. These experiments actually stimulated the septal area, which triggered dopamine release. Later experiments confirmed that it was, in fact, dopamine that mediated the effect of septal stimulation; if you inject a rat with dopamine-blocking drugs, the rat will no longer push a lever for septal stimulation (reviewed in Wise, 2008).
favor of dopamine stimulation: “A hungry rat often ignored available food in favor of the pleasure of stimulating itself electrically” (Olds, 1956).
(repeatedly removed from water): Kily et al., 2008.
such dopamine-enhancing chemicals: Cachat et al., 2010; Gerlai et al., 2000, 2009.
temporal difference learning signal: Schultz et al., 1997.
food in sixteen seconds: Kobayashi and Schultz, 2008.
“A Neural Substrate of Prediction and Reward”: Schultz et al., 1997.
monkey, and human brains: Grillner and Robertson, 2016; J. M. Li, 2012; Vindas et al., 2014.
for a failed prediction of future reward: For more information on this idea, see Joseph LeDoux’s great review of avoidance learning in LeDoux et al., 2017.
expected rewards or punishments: It can be hard to differentiate Pavlovian learning from learning from omission, and there are ongoing debates about the mechanisms of avoidance learning. For a great study showing that fish do truly learn from the omission of shock, see Zerbolio and Royalty, 1982.
five seconds after light: M. R. Drew et al., 2005; A. Lee et al., 2010. Reviewed in Cheng et al., 2011.
cycle of the day: Eelderink-Chen et al., 2021, shows circadian rhythm in prokaryotes; McClung, 2006, shows circadian rhythm in plants.
intervals between events: Abramson and Feinman, 1990; Craig et al., 2014. Reviewed in Abramson and Wells, 2018.
that maximize dopamine release: The connections between the cortex and basal ganglia contain different types of dopamine receptors. Rapid spikes of dopamine drive a strengthening between the cortical neurons and specific basal ganglia neurons that drive doing (or ungating) specific actions, and a weakening between cortical neurons and a different set of basal ganglia neurons that drive stopping (or gating) specific actions. Rapid declines of dopamine have the opposite effect. Through these parallel circuits, dopamine bursts reinforce recent behaviors and make them more likely to reoccur and dopamine declines punish behaviors and make them less likely to reoccur.
basal ganglia of dopamine: Cone et al., 2016, shows evidence that changing valence of sodium appetite derives from changes in input from lateral hypothalamus to midbrain dopamine neurons.
dopamine neurons directly: The purported actor circuit flows from the matrix of the striatum (the input structure of the basal ganglia); the purported critic circuit flows from the striosomes of the striatum.
Chapter 7: The Problems of Pattern Recognition
specific types of molecules: Niimura, 2009, provides evolutionary evidence that the olfactory receptors in modern vertebrates originated just before early vertebrates in the first chordates. Amphioxus (an animal often used as a model of the first chordates) has thirty-one functional vertebrate-like olfactory receptor genes and the lamprey (often used as a model for the early vertebrates) has thirty-two vertebrate-like olfactory receptor genes. Note that different lineages expanded the number of olfactory receptors; some modern fish have over one hundred, and rats have over one thousand.
As discussed in Niimura, 2012, while invertebrates also have olfactory receptors, their olfactory receptors seem to have independently evolved: “[Olfactory receptor] genes were also identified from other invertebrates including insects, nematodes, echinoderms, and mollusks . . . however, their evolutionary origins are distinct from that of vertebrate [olfactory receptor] genes. The neuroanatomical features of insect and vertebrate olfactory systems are common, but insect and vertebrate [olfactory receptor] genes are strikingly different to each other and share no sequence similarities.”
are similar but not the same: D. A. Wilson, 2009, and Laurent, 1999, proposed a similar model of olfactory coding. Barnes et al., 2008, found evidence of pattern completion in olfactory cortex of rats. Yaksi et al., 2009, found evidence of similar types of pattern separation and completion in fish. For one of the original papers suggesting that the three-layered cortex performs this type of auto-association, see Marr, 1971.
An artificial neural network: Picture from https://en.wikipedia.org/wiki/Artificial_neural_network#/media/File:Colored_neural_network.svg.
three layers of neurons: Teleost fish may not have clear layers, but the lamprey has a layered cortex, as do reptiles, hence I proceed with the assumption that the cortex of early vertebrates was layered (Suryanarayana et al., 2022).
networks to do math: McCloskey and Cohen, 1989. For reviews of current challenges in continual learning see Parisi et al., 2019, and Chen and Liu, 2018.
the year before: Brown, 2001.
learn this new pattern: Grossberg, 2012.
presented the cats with different visual stimuli: Hubel and Wiesel, 1959, 1962, 1968.
Figure 7.9: Figure from Manassi et al., 2013. Used with permission.
discovered by Hubel and Wiesel: Fukushima, 1980.
tap pictures to get food: Wegman et al., 2022.
rotated and translated objects: Worden et al., 2021. The cortex and thalamus are densely interconnected. It was originally believed that the thalamus was merely a “relay” of input to the cortex. But new research is beginning to call this into question. Three observations give us hints that the interactions between the thalamus and the cortex might be important for the invariance problem. First, most sensory input to the cortex flows through the thalamus—input from eyes, ears, and skin all first go to the thalamus and then branch out to various regions of the cortex. However, there is one exception to this rule: smell. The single sense that skips the thalamus and connects directly to the cortex is smell. Perhaps this is because smell is the only sense that does not have the invariance problem; it does not need to interact with the thalamus to recognize objects at different scales, rotations, and translations. Second, the thalamus and the cortex evolved together. Even in the most distant vertebrates, like the lamprey fish, there is both a cortex and a thalamus with similar interactions between them as in other vertebrates. This suggests that their function might be emergent from interactions between them. And third, the circuitry of the thalamus seems to precisely gate and route connections between different areas of the cortex.
Chapter 8: Why Life Got Curious
Montezuma’s Revenge: This story is retold in Christian, 2020.
to human infants: For curiosity in fish, see Budaev, 1997. For curiosity in mice, see Berlyne, 1955. For curiosity in monkeys, see Butler and Harlow, 1954. For curiosity in human infants, see Friedman, 1972.
no “real” reward: Matsumoto and Hikosaka, 2009.
cephalopods, show curiosity: Reviewed in Pisula, 2009. For cockroach curiosity, see Durier and Rivault, 2002. For curiosity in ants, see Godzinska, 2004. For cephalopod curiosity, see Kuba et al., 2006.
wasn’t present in early bilaterians: In the review on curiosity by Pisula, 2009, on page 48, he concludes that “parallels in exploratory behaviors must therefore be a result of convergence, i.e. a similar response to similar challenges presented by the environment, rather than common ancestry.”
Fish exhibit this effect as well: This partial reinforcement effect was found in goldfish in Wertheim and Singer, 1964. It was also shown in Gonzales et al., 1962, although with some differences from how the effect works in mammals.
Chapter 9: The First Model of the World
directly to the container with the food: Durán et al., 2008, 2010.
one thing relative to another thing: Larsch et al., 2015. For planarians see Pearl, 1903. For bees see Abramson et al., 2016.
through the entire loop again: Wehner et al., 2006.
Figure 9.1: Image by Carlyn Iverson / Science Source. Used with permission.
facing a certain direction: Bassett and Taube, 2001.
ventral cortex, and medial cortex: Note there is controversy around how to subdivide parts of the cortex in fish and amphibians. Some argue there are four areas, an additional one being the dorsal cortex (see Striedter and Northcutt, 2019).
cortex of early vertebrates: Note that the location of these areas of cortex is shifted in some modern vertebrates, which leads to different naming of the same functional structure, a complexity I have intentionally omitted for readability. In teleost fish, for example, the cortex does not fold in the same ways as in the lamprey, reptiles, and mammals (in teleost fish, the cortex goes through evagination, folding outward, instead of invagination, folding inward). So the location of the same functional structure (the hippocampal part of the cortex) ends up in different places in the brain, and thus the names anatomists give these areas differ. When I refer to the medial cortex of early vertebrates, I am referring to the functional structure in early vertebrates that would later become the hippocampus in mammals. In the lamprey and reptiles, this part of the cortex is the medial cortex, whereas in teleost fish, the identical part of the cortex is the lateral cortex. For simplicity I refer to this area of cortex as hippocampus and graphically represent the cortex only in its invaginated state (hence the hippocampus structure shows up in the medial part), since we use the lamprey as the model organism for early vertebrates.
are facing specific directions: Fotowat et al., 2019; Vinepinsky et al., 2020.
a spatial map: Petrucco et al., 2022, shows evidence of head-direction cells in the hindbrain of fish. For a review of the overall network of head-direction cells and input to the hippocampus in rodents, see Yoder and Taube, 2014.
ability to remember locations: Broglio et al., 2010; Durán et al., 2010; López et al., 2000; Rodríguez et al., 2002.
turn in a maze: Rodríguez et al., 2002.
space to get food: Durán et al., 2010.
given different starting locations: Broglio et al., 2010.
similarly impairs spatial navigation: Naumann and Laurent, 2016; Peterson, 1980; Rodríguez et al., 2002.
Chapter 10: The Neural Dark Ages
to previously inhospitable areas: Algeo, 1998.
were thirty meters tall: Beck, 1962.
The Late Devonian Extinction: Algeo et al., 1995; McGhee, 1996.
than you can underwater: Mugan and MacIver, 2020.
and plan are birds: Boeckle and Clayton, 2017.
Figure 10.3: I am leaving out the dorsal cortex from early vertebrates because there is still debate about its presence (Striedter and Northcutt, 2019). For the alignment between medial cortex to hippocampus, lateral cortex to olfactory cortex, and ventral cortex to associative amygdala, see Luzzati, 2015; Striedter and Northcutt, 2019.
Chapter 11: Generative Models and the Neocortical Mystery
stimuli elicited what responses: Talbot et al., 1968.
test of Mountcastle’s hypothesis: von Melchner et al., 2000.
Figure 11.4: “Editor” image from Jastrow (1899). Other images from Lehar S. (2003), obtained from Wikipedia.
Figure 11.5: Staircase from Schroeder (1858). “Necker cube” from Louis Necker (1832). Duck or rabbit from Jastrow (1899).
Figure 11.6: Image from Fahle, et al., 2002. Used with permission by The MIT Press.
with Helmholtz’s theory: Later incarnations of Helmholtz’s ideas include analysis-by-synthesis (Neisser, 1967) and Mackay’s epistemological automata (MacKay, 1956).
the Helmholtz machine: Dayan, 1997; Hinton et al., 1995.
learned on its own: Dayan, 1997; Ibid.
Figure 11.8: Image from Hinton et al., 1995. Used with permission.
thispersondoesnotexist.com: Uses a StyleGAN2 (Karras et al., 2020).
Figure 11.10: Figure from He et al., 2019. Used with permission.
with a generative model: Reichert et al., 2013.
“constrained hallucination”: Seth, 2017.
presence of REM sleep: The only nonmammalian animals shown to also have mammal-like sleep states of alternated NREM and REM sleep (suggestive of dreaming) are birds (Johnsson et al., 2022; Lesku and Rattenborg, 2014; Rattenborg et al., 2022).
process of forced generation: For a nice overview of theories of why we dream, the connection to generative models, and alternative explanations for dreaming, see Prince and Richards, 2021.
processing actual visual data: van der Wel and van Steenbergen, 2018.
imagine the same thing: O’Craven and Kanwisher, 2000.
moving the body parts: Doll et al., 2015.
(recording their brains): For a good review, see Pearsona and Kosslynb, 2015. There is now strong evidence that when one visualizes, there is activity in area V1 (Albers et al., 2013; Slotnick et al., 2005; Stokes et al., 2009). For evidence that one can decode imagery from activation in visual neocortex, see Kay et al., 2008; Naselaris et al., 2015; Thirion et al., 2006.
(the left visual field): Bisiach and Luzzatti, 1978; Farah et al., 1992.
that unfolds over time: Jeff Hawkins has some great writing about this. See his books J. Hawkins, 2021, and J. Hawkins, 2004.
“from AI systems today”: tweeted by Yann LeCun (@lecun) on December 19, 2021.
Chapter 12: Mice in the Imaginarium
rotated, rescaled, or perturbed: Volotsky et al., 2022.
before choosing a direction: Tolman, 1939, 1948.
try the next option: Steiner and Redish, 2014. As reviewed in Redish, 2016.
to consider alternative paths: Schmidt et al., 2013.
turning right and getting: Ibid.
the barrier at all: Beyiuc, 1938 (page 409), describes this unfolding in a betta fish. Gómez-Laplaza and Gerlai, 2010, is the only counterexample I am aware of where fish were reported to do latent learning of a map and make the right decision ahead of time. It is hard to interpret a single counterexample without further replications. But if it turns out that some fish can, in fact, perform latent learning, then this suggests either that early vertebrates could solve latent-learning tasks without planning, planning evolved independently in some fish, or planning in some form existed in early vertebrates.
navigate around a barrier: Lucon-Xiccato et al., 2017.
runs toward the salt: Tindell et al., 2009.
they called “restaurant row”: Steiner and Redish, 2014. As reviewed in Redish, 2016. Also see Bissonette et al., 2014.
play rock, paper, scissors: Abe and Lee, 2011.
did not occur either: D. Lewis, 1973.
(of reasoning in birds): For evidence of causal reasoning in rats, see Blaisdell et al., 2006; M. R. Waldmann et al., 2012. Fischer and Schloegl, 2017, conclude that causal reasoning evolved in early mammals and independently evolved also in birds.
you’re remembering the past: Addis et al., 2007.
(saw with imagining things): O’Craven and Kanwisher, 2000; J. Pearson et al., 2015.
mistaken eyewitness testimony: Shermer et al., 2011.
the event did occur: Garry et al., 1996.
to get more food: Crystal, 2013; W. Zhou et al., 2012.
structures for rendering simulations: Many neuroscientists don’t like using the term episodic memory to refer to the process by which these simpler mammals, such as rats, recall past life events. The term episodic memory has been loaded with a baggage of concepts, such as the conscious experience of mentally time traveling or some notion of an autobiographical self. Many neuroscientists instead use the safer term episodic-like memory. Indeed, it isn’t clear how experientially rich the recollections of rats are. But regardless, the evidence from rats suggests that the precursor to episodic memory was present in early mammals.
state and the best actions: Actions include not just motor movements but the “next target location in space.” In other words, the “spatial map” of ancestral vertebrates is not considered model-based RL because it doesn’t seem to be employed for the purpose of simulating future actions. But it could still be employed for place recognition and the construction of homing vectors.
games were model-free: Mnih et al., 2013.
are model-free: Wang, 2018.
Chapter 13: Model-Based Reinforcement Learning
complex games like chess: Baxter et al., 2000, offers a TD-learning approach to playing chess (which still uses tree search and hence is not model-free) and provides a nice summary of the struggles of using model-free approaches (no search) in chess.
Go champion Lee Sedol: Silver et al., 2016.
Go than in chess: M. James, 2016.
The game of Go: Figure from https://en.wikipedia.org/wiki/Go_(game)#/media/File:FloorGoban.JPG.
board position in Go: AlphaGo documentary (Kohs, 2020).
(possible subsequent next moves): Ibid.
(body and navigational paths): Ibid.
about anything at all: Devinsky et al., 1995; Németh et al., 1988; B. A. Vogt, 2009.
“no ‘will’ to reply”: Damasio and van Hoesen, 1983.
to be a mammal: According to J. H. Kaas, 2011, early mammals had two main areas of frontal cortex: anterior cingulate and orbitofrontal cortex. When I refer to agranular prefrontal cortex of early mammals, I am referring to both of these regions. The anterior cingulate cortex in humans is considered homologous to the prelimbic, infralimbic, dorsal anterior cingulate cortex of rodents (Laubach et al., 2018; van Heukelum et al., 2020), all of which can be assumed to have been inherited from the anterior cingulate cortex of the first mammals.
vicarious trial and error: Lose head toggling behavior: Schmidt et al., 2019; lose goal representations in hippocampus: Ito et al., 2015.
episodic-memory recall: Frankland et al., 2004.
or counterfactual learning: J. L. Jones et al., 2012.
effort is worth it: Friedman et al., 2015.
to repeat past mistakes: Frankland et al., 2004.
repeat already completed actions: Goard et al., 2016; Kamigaki and Dan, 2017; Kopec et al., 2015.
patient to get food: Inactivating prelimbic area of rat frontal cortex (part of their aPFC) increases premature responses (e.g., lever-release before go stimulus). (Hardung et al., 2017; Narayanan et al., 2006). Inactivation of the aPFC (prelimbic and infralimbic cortex) in rats increases impatient trials and decreases waiting time (Murakami et al., 2017). For a good review on the role of prefrontal areas in behavioral inhibition, see Kamigaki, 2019. Also see M. G. White et al., 2018.
toward an imagined goal: Procyk et al., 2000; Procyk and Joseph, 2001. The latter observed that neurons in the monkey anterior cingulate cortex (a part of aPFC) are sensitive to the order of actions executed in a sequence (even if the actual movements performed are the same), suggesting that this area of the brain is modeling the overall sequence an animal is within, not just individual movements. There is also evidence for this in rats (Cowen et al., 2012; Cowen and McNaughton, 2007) and humans (Koechlin et al., 2002).
in an ongoing task: Dehaene et al., 1994; MacDonald et al., 2000; Ridderinkhof et al., 2004; Totah et al., 2009. For an interesting synthesis, also see Shenhav et al., 2016.
is reported to be: For some further reading on this, see Gal, 2016, and Lakshminarayanan et al., 2017.
of the basal ganglia: The frontal cortex sends a direct projection to a part of the basal ganglia called the subthalamic nucleus that has been shown to be able to completely halt behavior (Narayanan et al., 2020).
with levels of uncertainty: E. R. Stern et al., 2010.
activity of the sensory neocortex: For a good review of this circuitry, see Kamigaki, 2019. Note that different frontal regions may be associated with different sensory modalities—some subregions of the aPFC (like the anterior cingulate cortex) mostly send output to visual and not somatosensory and auditory areas, whereas others mostly send output to auditory and somatosensory areas (S. Zhang et al., 2016).
cortex become uniquely synchronized: Benchenane et al., 2010; Spellman et al., 2015, show synchronization between the aPFC and hippocampus during vicarious trial and error, as reviewed in Redish, 2016. Hyman et al., 2010; M. W. Jones and Wilson, 2005, show synchronization between the hippocampus and PFC during episodic memory tasks. Sauseng et al., 2004; Sederberg et al., 2003; Xie et al., 2021, show this same synchronization between prefrontal and sensory neocortex during working memory and episodic-memory tasks.
an action is selected: Bogacz and Gurney, 2007; Krajbich et al., 2010.
those that had not: Dickinson, 1985.
the food was devalued: Ibid.
calls this “active inference”: Adams et al., 2013.
missing the fourth layer: His theory was about the motor cortex, which is also agranular (see next chapter), but the logic applies equally to aPFC.
(looking at the sky): There is some evidence that neocortical columns oscillate between different states when different layers are suppressed and they oscillate at different rhythms when you’re imagining versus when you’re attending to things. I review the evidence in Bennett, 2020.
duck or a rabbit: S. Zhang et al., 2014, shows frontal circuits for modulating representations in the sensory neocortex.
lizards hundreds of trials: Wagner, 1932.
if you damage a rat’s aPFC: Dias and Aggleton, 2000. Note that rats can still learn non-matching-to-position tasks at approximately normal rates because they do not have to overcome their instinctual tendency to avoid recently exploited foraging sites (avoiding the place they just experienced is instinctual). Discussed in Passingham and Wise, 2015.
Chapter 14: The Secret to Dishwashing Robots
cortex damage: Darling et al., 2011.
other areas of the neocortex: Although note that the motor cortex is “agranular,” meaning it has a thin or missing layer four (just like agranular prefrontal cortex).
evolution of the motor cortex: Karlen and Krubitzer, 2007.
bats, elephants, and cats: Karlen and Krubitzer, 2007. The placental-marsupial divergence is believed to have occurred about 160 million years ago in the Jurassic period (Z. X. Luo et al., 2011).
suffer from such paralysis: Kawai et al., 2015; Whishaw et al., 1991. Neurons in the motor cortex of primates bypass older circuits and make direct connections with spinal neurons (Lemon, 2019). Although some evidence suggests such direct projections may also occur in rats (Elger et al., 1977; Gu et al., 2017; Maeda et al., 2016), new evidence shows that these direct projections disappear in adulthood (Murabe et al., 2018), unlike in primates (Armand et al., 1997; Eyre, 2007). The paralysis of motor-cortex damage in primates does not seem to be representative of the motor cortex in early mammals.
small unevenly placed platform: Alaverdashvili and Whishaw, 2008; T. Drew et al., 2008; T. Drew and Marigold, 2015; Grillner and el Manira, 2020.
learn the lever sequence: Kawai et al., 2015.
Figure 14.3: Art by Rebecca Gelernter; this particular figure was inspired by imagery from Grillner and el Manira, 2020.
movements that require planning: Beloozerova et al., 2010; Farrell et al., 2014; Grillner and el Manira, 2020.
known to be there: Andujar et al., 2010; Beloozerova and Sirota, 2003; T. Drew and Marigold, 2015.
movement it presumably planned: Lajoie et al., 2010.
motor cortex becomes activated: Malouin et al., 2003.
unrelated to movement: Kosonogov, 2011.
and even surgical maneuvers: Arora et al., 2011.
the whole affair is: Kohlsdorf and Navas, 2007; Olberding et al., 2012; Parker and McBrayer, 2016; Tucker and McBrayer, 2012.
to get around platforms: Kohlsdorf and Biewener, 2006; Olberding et al., 2012; Self, 2012.
can cure drug addiction: N. Li et al., 2013.
lower-level subgoals: Lashley, 1951; Yokoi and Diedrichsen, 2019.
levels in the hierarchy: Thorn et al., 2010.
(trials they go through): Yin et al., 2004.
own without their control: Brainin et al., 2008.
damage to the premotor cortex: P. Gao et al., 2003.
that nearby stimuli suggest: Lhermitte, 1983.
rate dropped to 42 percent: N. Li et al., 2013.
Chapter 15: The Arms Race for Political Savvy
of land-living vertebrates: Sahney and Benton, 2008.
Figure 15.2: Figure from ibid.
bigger its social group: Dunbar, 1998.
for most other animals: Pérez-Barbería et al., 2007; Shultz and Dunbar, 2007. Reviewed in Dunbar and Shultz, 2017.
down and look away: Stringham, 2011.
and flatten their ears: S. Curtis, 1998.
multi-male groups: Shultz and Dunbar, 2007. Original data from Nowak, 1999. Note these classifications are inexact, not all subspecies fall into one or the other, and there may be other categorizations as well. But these are the common ones and are the broad first approximations of different types of social organizations. Dunbar used these four categorizations in some of his seminal work, and they are standard in primate literature (B. B. Smuts et al., 1987).
four common social structures found in mammals: Shultz and Dunbar, 2007. Original data from Nowak, 1999.
his own children: While most harem-organizing mammals have a single male and multiple females, there are indeed cases where the roles are reversed. Some marmosets, monkeys, and marsupials have single females that mate with multiple males (Goldizen, 1988). And of course, in many insects, like bees, female-run social groups are less the exception and more the rule.
avoidance of large groups: R. A. Hill and Dunbar, 1998.
they created in response: Bettridge et al., 2010.
living in a one-acre forest: Menzel, 1974. Story summarized in Kirkpatrick, 2007.
“accidental” and “intentional” actions: Call and Tomasello, 1998.
those who seemed unwilling: Call et al., 2004.
goggles wouldn’t see them: Kano et al., 2019.
like some birds, dolphins: Tomonaga, 2010.
location the trainer knows about: Bräuer, 2014; Kaminski et al., 2009.
grooming and group size: Dunbar, 1991, 1998; Lehmann et al., 2007.
by grooming themselves more: R. M. Seyfarth, 1980.
by appearance and voice: Snowdon and Cleveland, 1980.
what the mother does: D. L. Cheney and Seyfarth, 1980, 1982.
A will submit to C: Andelman, 1985; R. M. Seyfarth, 1980.
many years, even generations: D. Cheney, 1983.
play back these recordings: Bergman et al., 2003.
on and so forth: Berman, 1982; Horrocks and Hunte, 1983; Walters and Seyfarth, 1987.
rank of her mother: Berman, 1983; Lee, 1983.
of higher-ranking families: Datta, 1983.
to die from disease: Silk, 1987; Silk et al., 2003, 2010.
hierarchy has been established: M. R. A. Chance et al., 1977; Gouzoules, 1980.
nonfamily members that come: Reviewed in chapter 2 of D. L. Cheney and Seyfarth, 2019.
to recruit such allies: Chapais, 1988; also discussed in chapter 2 of D. L. Cheney and Seyfarth, 2019.
formed grooming partnerships with: D. Cheney, 1983.
makes a “help me” vocalization: R. M. Seyfarth and Cheney, 1984.
to their own defense: F. B. M. de Waal, 1982; Packer, 1977; B. B. Smuts, 2017.
food just for themselves: Engelmann and Herrmann, 2016.
to deal with Keith: Datta, 1983; Dunbar, 2012.
more access to food: Packer, 1977; Silk, 1982.
ranked higher than themselves: Cheney and Seyfarth, 2019.
members of the group: Gouzoules, 1975; Scott, 1984.
with high-ranking individuals: D. L. Cheney and Seyfarth, 2019.
the higher-ranking individual: D. Cheney, 1983; D. L. Cheney, 1977; R. M. Seyfarth, 1977.
the most popular playmates: P. Lee, 1983.
you under my wing: Stammbach, 1988b, 1988a.
with nonfamily members: Cheney and Seyfarth, 1989.
have recently quarreled with: Cheney and Seyfarth, 2019.
eating, resting, and mating: Dunbar et al., 2009.
than most other mammals: Dunbar, 1991.
spend more time socializing: Borgeaud et al., 2021.
with social savviness: Byrne and Corp, 2004.
Chapter 16: How to Model Other Minds
about three hundred fifty grams: Ginneken et al., 2017; Tobias, 1971; van Essen et al., 2019.
the same fundamental ways: To be fair, primates do have a higher density of neurons in their neocortices, but this denser packing doesn’t suggest a change to the overall architecture of a neocortical column, merely that it has been scaled up and packed into a smaller area. For a good review of brain scaling, see Herculano-Houzel, 2012.
addition to the frontal cortex: Preuss, 2009.
and sensory neocortical regions: Goldman-Rakic, 1988; Gutierrez et al., 2000.
functional significance at all: Hebb, 1945; Hebb and Penfield, 1940; H. L. Teuber and Weinstein, 1954.
intellect or perception whatsoever: Hebb and Penfield, 1940.
cortex was a “riddle”: H. Teuber, 1964.
gPFC lit up: Gusnard et al., 2001.
about yourself in general: Christoff et al., 2009; Herwig et al., 2010; Kelley et al., 2002; Moran et al., 2006; Northoff et al., 2006; Schmitz et al., 2004.
of the surrounding elements: Kurczek et al., 2015.
themselves in a mirror: Breen et al., 2001; Postal, 2005; Spangenberg et al., 1998.
the amygdala and hippocampus: Morecraft et al., 2007; Insausti and Muñoz, 2001.
from the older aPFC: Ray and Price, 1993. Further evidence for this is seen in the fact that stimulation of the agranular cortex in primates elicits autonomic effects (changes in respiratory rate, blood pressure, pulse, pupillary dilation, and piloerection), while in the granular cortex, it does not (Kaada, 1960; Kaada et al., 1949).
lit up with activation: Brunet et al., 2000; Völlm et al., 2006. The comic-strip task is similar to the earlier storytelling work by Baron-Cohen et al., 1986.
Figure 16.4: Images from Brunet et al., 2000; Völlm et al., 2006; and personal correspondence with Dr. Eric Brunet-Gouet. Used with permission of Dr. Brunet-Gouet (personal correspondence).
the age of four: H. M. Wellman et al., 2001; H. Wimmer and Perner, 1983.
Figure 16.5: Photo from Frith, 2003. Reused with permission.
the degree of activation: Gweon et al., 2012; Otsuka et al., 2009; Saxe and Kanwisher, 2003; Young et al., 2007.
such false-belief tasks: Carrington and Bailey, 2009; van Overwalle and Baetens, 2009, implicate specifically two areas of the granular prefrontal cortex (dorsomedial prefrontal cortex and anteromedial prefrontal cortex, which roughly make up Brodmann areas 8, 9, and 10) as well as the temporoparietal junction and the superior temporal sulcus as areas that are uniquely activated by tasks that require the theory of mind.
the Sally-Ann test: Siegal et al., 1996; V. E. Stone et al., 1998.
emotions in other people: Shaw et al., 2005.
with other people’s emotions: Shamay-Tsoory et al., 2003.
distinguish lies from jokes: Winner et al., 1998.
that would offend someone: Shamay-Tsoory et al., 2005; V. E. Stone et al., 1998.
someone else’s visual perspective: Stuss et al., 2001.
struggle to deceive others: Ibid.
effects in nonhuman primates: Dehaene et al., 2005; D. I. Perrett et al., 1992; Ramezanpour and Thier, 2020.
it does in humans: T. Hayashi et al., 2020.
social-network size in primates: Sallet et al., 2011.
theory-of-mind tasks: J. Powell et al., 2012; Stiller and Dunbar, 2007; P. A. Lewis et al., 2011; J. L. Powell et al., 2010.
to model other minds: See Amodio and Frith, 2006, for a detailed review of the specific areas in the prefrontal cortex implicated in self-reference and thinking about others.
or “social projection theory”: Gallese and Goldman, 1998; Goldman, 1992; Gordon, 2011; Harris, 1992. It should be noted that not everyone agrees that these are implemented by the same process. For some good reviews of this debate, see Dimaggio et al., 2008; Gallup, 1998.
the same process: When evaluating your own personality traits or receiving evaluations of yourself by others, the same mentalizing network in the gPFC activates—specifically, the medial area of the prefrontal cortex (Ochsner et al., 2005).
of theory of mind: Further consistent with this idea that theory of mind of others is bootstrapped on a generative model of yourself is the fact that the concept of self emerges in child development before the theory of mind emerges. See Keenan et al., 2005; Ritblatt, 2000; Rochat, 1998.
about two years old: Amsterdam, 1972.
want, wish, and pretend: Frith and Frith, 2003.
“know it’s a crocodile”: Shatz et al., 1983.
with respect to other people: H. M. Wellman et al., 2001.
better at the other: Gopnik and Meltzoff, 2011; Lang and Perner, 2002.
themselves in a mirror: Gallup et al., 1971. There is some evidence suggesting that elephants and dolphins can recognize themselves in a mirror (Plotnik et al., 2006; Reiss and Marino, 2001). Recognition is seen in apes, such as chimps, orangutans, and gorillas (Suarez and Gallup, 1981; Posada and Colell, 2007). Monkeys may also recognize themselves in a mirror (Chang et al., 2017). A great summary of these mirror tests can be found in chapter 3 of Suddendorf, 2013.
these states in others: Kawada et al., 2004; Niedenthal et al., 2000.
thirstier than they are: van Boven and Loewenstein, 2003.
personality traits onto others: Bargh and Chartrand, 2000.
the minds of others: Michael Graziano has some fascinating ideas about this and its relationship to consciousness. He argues that our ancestors evolved theory of mind to navigate their unique social lives and a side effect of this was that when they applied this theory of mind inward, consciousness emerged (Graziano, 2019).
(“This is jumping”): Surís et al., 2021.
(faces classified by emotions): Crawford, 2021.
Chapter 17: Monkey Hammers and Self-Driving Cars
to clean their ears: Flossing: Pal et al., 2018. Lists of different techniques: Sanz and Morgan, 2007.
flies and scratch themselves: Hart et al., 2001.
to break open nuts: Müller, 2010.
to the inner food: Bernardi, 2012.
different tool-using behaviors: Sanz and Morgan, 2007.
than those in Gombe: Musgrave et al., 2020.
and others for tearing: di Pellegrino et al., 1992.
had just happened: Story told in Taylor, 2016 and Roche and Commins, 2009.
(sticking one’s tongue out): di Pellegrino et al., 1992; Ferrari et al., 2003; Gallese et al., 1996.
(parietal lobe, motor cortex): di Pellegrino et al., 1992; Dushanova and Donoghue, 2010; Fogassi et al., 2005; Tkach et al., 2007.
numerous species of primates: Brass et al., 2007; Buccino et al., 2001; Mukamel et al., 2010.
interpretations of mirror neurons: For reviews of the current debate around mirror neurons, see Heyes and Catmur, 2022; Hickok, 2014; Jeon and Lee, 2018; Rozzi, 2015.
in theory of mind: Rizzolatti et al., 2001.
see someone else do: Gallese and Goldman, 1998.
open (without seeing anything): Kohler et al., 2002.
(box behind the wall): Umiltà et al., 2001.
(phrase, like combing hair): Pazzaglia et al., 2008; Tarhan et al., 2015; Urgesi et al., 2014.
bouncing on its own: Pobric and Hamilton, 2006. Consistent with this, if people actively pick up a light box, they become biased toward thinking that boxes they see others pick up are also light. This bias is far greater when an individual actually lifts the box as opposed to passively holding a light box, which demonstrates it isn’t about associating a box with lightness but about the active experience of picking up a box yourself (A. Hamilton et al., 2004).
(eating a burger, blowing out a candle): Michael et al., 2014.
intended to hold: Thompson et al., 2019, provides a nice review of some of these ideas. But see Negri et al., 2007 and Vannuscorps and Caramazza, 2016 for counterexamples that suggest that impairments to action production don’t always impair action perception.
becomes way more activated: S. Vogt et al., 2007.
following the red dots: Catmur et al., 2009; Heiser et al., 2003.
learns to do it: Humle et al., 2009; Lonsdorf, 2005.
skill later in life: Biro et al., 2003; Matsuzawa et al., 2008.
(food in the cage): M. Hayashi et al., 2005; Marshall-Pescini and Whiten, 2008; Tomasello et al., 1987; Subiaul et al., 2004.
in the right way: Whiten et al., 2005.
way to get food: Dindo et al., 2009.
drawer to get food: Gunhold et al., 2014.
opening an artificial fruit: E. van de Waal et al., 2015.
down through multiple generations: Haslam et al., 2016; Mercader et al., 2007; Whiten, 2017.
lever and get water: Zentall and Levine, 1972.
technique of their parents: Müller and Cant, 2010.
other dolphins or humans: Hermann, 2002.
dog perform the act: Range et al., 2007.
those same navigational paths: For observational learning in fish, see Lindeyer and Reader, 2010. For reptiles, see Kis et al., 2015; Wilkinson, Kuenstner, et al., 2010; Wilkinson, Mandl, et al., 2010.
actively teach one another: For arguments on this, see Hoppitt et al., 2008; Kline, 2014; Premack, 2007.
hands of their young: Boesch, 1991.
down to help teach: Masataka et al., 2009.
swap tools with them: Musgrave et al., 2016.
tool to a youngster: Musgrave et al., 2020.
skipped the irrelevant steps: Call et al., 2005; Horner and Whiten, 2005; Nagell et al., 1993.
a handful of laps: Story told in Christian, The Alignment Problem, 232.
called “inverse reinforcement learning”: Abbeel, Coates, and Ng, 2004.
a remote-controlled helicopter: Abbeel et al., 2010.
imitation learning in robotics: For a nice review of challenges in inverse reinforcement learning, see Hua et al., 2021.
Chapter 18: Why Rats Can’t Go Grocery Shopping
less than seventy-two hours: Milton, 1981.
a less competitive fruit: Janmaat et al., 2014.
to be depleted more quickly: Noser and Byrne, 2007.
evolve to survive winters: Barry, 1976.
140 species of primates: DeCasien et al., 2017.
the “Bischof-Kohler hypothesis”: Suddendorf and Corballis, 1997.
before they were cold: F. B. M. de Waal, 1982.
of that task: Mulcahy and Call, 2006.
have no suitable stones: Boesch and Boesch, 1984.
use in another location: Goodall, 1986.
change their behavior accordingly: Naqshbandi and Roberts, 2006.
“hunger will be satisfied”: Suddendorf and Corballis, 1997.
and anticipating future needs: Note that Suddendorf is still skeptical of studies suggesting that other animals can anticipate future needs (personal correspondence). In fact, Suddendorf is skeptical about whether any animals other than humans are capable of considering the future at all (see Suddendorf, 2013; Suddendorf and Redshaw, 2022). His fascinating book The Invention of Tomorrow describes his argument.
that were well fed: Mela et al., 1996; Nisbett and Kanouse, 1969.
Summary of Breakthrough #4: Mentalizing
at similar developmental times: Children seem to begin to anticipate future needs around age four (Suddendorf and Busby, 2005), which is same age that they begin to pass theory of mind tasks (H. M. Wellman et al., 2001). For a review of different theories of mental time travel, see Suddendorf and Corballis, 2007.
ago in eastern Africa: T. D. White et al., 2009.
Chapter 19: The Search for Human Uniqueness
“and not of kind”: Darwin, 1871.
in all the same ways: Herculano-Houzel, S. 2009
sounds and gestures: Graham and Hobaiter, 2023; Hobaiter and Byrne, 2014; Hobaiter and Byrne, 2011.
signed, Finger bracelet: “Mission Part 1: Research.” Koko.org.
running from his trainer: L. Stern, 2020.
70 percent of the time: Savage-Rumbaugh et al., 1993.
(I want to be tickled): Yang, 2013, compared the diversity of phrases between young children and the chimpanzee Nim Chimpsky. Yang, 2013, concluded that the children showed the level of diversity consistent with the use of grammar to construct novel phrases but Nim Chimpsky did not, thus concluding that Chimpsky’s phrase diversity was more consistent with directly memorizing phrases.
few noises or gestures: Some of my favorite writing on this is Daniel Dor’s book The Instruction of Imagination (New York: Oxford University Press, 2015).
“money paid out in fees”: Harari, 2015.
on different cooperation strategies: Dunbar, 1993, estimated human group size at 150 (the famous Dunbar’s number) by looking at the human neocortex ratio and examining tribal societies. B. B. Smuts et al., 1987, report approximately 50 as an average group size in chimpanzees and approximately 18 as an average group size in capuchin monkeys.
be about one hundred fifty people: Dunbar, 1992, 1993.
even millions, of generations: For reviews of the theory of cumulative culture see Tennie et al., 2009; Tomasello et al., 1993.
as one hundred thousand years ago: Toups et al., 2011.
children are over-imitators: D. E. Lyons et al., 2007.
copy all the steps: For example, Gergely et al., 2002, and Schwier et al., 2006, showed that twelve- and fourteen-month-old infants are more likely to copy an unusual component of a sequence when it was not clear why it was done and less likely to copy it if the teacher was “forced” to do the unusual action due to some physical limitation. For example, Schwier et al., 2006, had teachers demonstrate putting a toy dog into a toy house that had two openings, one through a front door and another through a chimney. In cases where the front door was blocked and the teacher demonstrated putting the dog into the toy house through the chimney, infants were less likely to do the same when it was their turn (if the door was open for them); they just put it through the door (achieving the same goal through different means). In contrast, when teachers put the dog into the house through the chimney when the door was open (hence the teacher clearly chose the chimney for some reason), infants did the same and put it through the chimney.
pull the toy apart: Carpenter et al., 1998; Meltzoff, 1995.
improves the accuracy and speed: Chopra et al., 2019; Dean et al., 2012.
discontinuity that changed everything: My favorite writings about the idea of cumulative culture are in Tennie et al., 2009.
can persist across generations: Henrich, 2004.
Chapter 20: Language in the Brain
“like you want before”: Aphasia. National Institute on Deafness and Other Communication Disorders. March 6, 2017. Accessed on March 5, 2023 at https://www.nidcd.nih.gov/health/aphasia.
to understand speech: For a review of Wernicke’s area, see DeWitt and Rauschecker, 2013.
selective for language in general: Campbell et al., 2008.
are in writing words: Chapey, 2008.
Broca’s area is damaged: Emmorey, 2001; Hickok et al., 1998; Marshall et al., 2004.
spoken language and written language: DeWitt and Rauschecker, 2013; Geschwind, 1970.
watches someone sign: Neville et al., 1997.
are otherwise intellectually typical: Lenneberg, 1967, showed that language capacity is radically dissociated from other cognitive capacities. For a more recent review, see Curtiss, 2013.
over fifteen languages: Smith and Tsimpli, 1995.
a scaled-up chimpanzee brain: Herculano-Houzel, 2012; Herculano-Houzel, 2009, show that the human brain is largely just a scaled-up primate brain. Semendeferi and Damasio, 2000, show that the prefrontal cortex of humans is not uniquely enlarged relative to other primates (it was just scaled up proportionally with the rest of the brain).
The few differences that have been found in human brains relative to other primate brains include the following. First, humans have a unique projection from the motor cortex to the area of the larynx that controls the vocal cords. So, yes, humans have unique control over their voice boxes, and this is clearly related to speech. But as we review later in the chapter, this is not what unlocked language, for there are many nonvocal languages that are as sophisticated without ever using this projection (such as the sign languages of people born deaf). Second, although there are no uniquely human areas of neocortex (all the same areas are found in other primates), there is indeed some evidence that the relative space devoted to different areas of prefrontal cortex might be somewhat different in humans (Teffer and Semendeferi, 2012). And third, minicolumns in the neocortex of humans might have greater width than those in other primates (Buxhoeveden and Casanova, 2002; Semendeferi et al., 2011), although this doesn’t prove that there is anything fundamentally different about the neocortical microcircuit itself in humans. But if it were at some point discovered that the neocortical microcircuit of humans was, in fact, fundamentally wired differently (it would have to be a subtle enough change that it has eluded our detection thus far), this would surely require us to rethink this entire evolutionary story, as it would open the door for the possibility that the human neocortex does enable something that is different “in kind.”
impact on monkey communication: Aitken, 1981; Jürgens, 1988; Jürgens et al., 1982.
controlled by the neocortex: Burling, 1993.
Figure 20.2: Image from Trepel et al., 1996, used with permission.
“impossible task [for chimpanzees]”: Goodall, 1986.
system in the neocortex: Note that this does not mean that emotional states themselves emerge only from the amygdala and brainstem. It merely means that the automatic emotional expressions—the smiles, frowns, and cries—are hardwired into these circuits. But emotional experiences and states in humans (and other mammals) are more complicated and do probably involve the cortex.
normal gesture-call behavior: Hammerschmidt and Fischer, 2013.
of the same gestures: Graham et al., 2018.
to similar emotional states: The extent to which emotional expressions and corresponding emotional states are universal versus culturally learned is controversial. Attempts to define explicit emotion categories using facial expressions has recently been challenged, as it turns out that much of what people define as one category in one culture does not always translate to another. I do not mean to suggest that emotions like anger and happy are universal. But even if many aspects of emotion categories are learned, this does not mean that there is no initial template with predefined emotional expressions that humans are born with. Indeed, I am unaware of any reports of a child who screams and cries in response to happy things and smiles and laughs in response to painful things. Babies born both deaf and blind still smile, laugh, frown, and cry normally (Eibl-Eibesfeldt, 1973). As mammals develop, the neocortex learns, and it can modulate and modify genetically prewired systems in the midbrain and hindbrain and thus complexify, modify, and expand on the emotional-expression template that we are born with. This is a general way mammal brains develop. For example, the midbrain and hindbrain of a baby have pre-defined wiring for basic motor behaviors (grasping). As the neocortex learns, it begins to take over and modulate these midbrain and hindbrain circuits to override them and take control over the hands. But this does not mean that there was not already a hardwired circuit for grasping.
For the evidence showing some universality in human emotional expressions across cultures, see Ekman, 1992; Ekman et al., 1969; Ekman and Friesen, 1971; Scherer, 1985. For evidence that emotion categories are not as universal as previously thought, see Lisa Feldman Barrett’s wonderful book How Emotions Are Made and Barrett et al., 2019. Barrett’s theory of constructed emotion, whereby the neocortex constructs emotion categories, is consistent with the idea presented in this book whereby the aPFC and gPFC construct a generative model of the self and construct explanations of an animal’s own behaviors and mental states. An emergent property of this might be constructing the notion of an emotional state—using the notion of anger to explain the behavioral repertoire the animal performing. (Similar, or perhaps the same, to how we have suggested the aPFC constructs intent.)
it later in life: Andrei, 2021.
but will never speak: Lenneberg, 1967.
given the previous words: He introduced the simple recurrent neural network, also called the Elman network (Elman, 1990).
as the model itself: For a well-written discussion on curriculum, see Christian, 2020.
facial expressions, and gestures: Beebe et al., 1988, 2016.
joint attention to objects: Tomasello, 1995.
back at her mother: Carpenter and Call, 2013.
and point again: Liszkowski et al., 2004.
same object they do: Warneken et al., 2006.
vocabulary is twelve months later: Morales et al., 2000; Mundy et al., 2007.
simplest questions about others: Hauser et al., 2002.
another’s inner mental world: Some claim that some nonhuman apes in these studies did ask some types of questions. It is still controversial.
when asking yes/no questions: D. L. Everett, 2005; Jordania, 2006.
of our language curriculum: MacNeilage, 1998; Vaneechoutte, 2014.
Chapter 21: The Perfect Storm
Figure 21.1: DeSilva et al., 2021.
“runaway growth of the brain”: D. Everett, 2017, 128.
on which the forest depended: Davies et al., 2020.
would eventually become human: Coppens, 1994. Although it is undisputed that the climate changed in Africa around ten million years ago, how dramatic this change was and how relevant it was to our ancestors becoming bipedal is not settled.
legs instead of four: Set of cranial volumes from different fossils: Du et al., 2018.
of a modern chimpanzee’s: Bipedalism appeared before tool use as well (see Niemitz, 2010).
was scavenging carcasses: Bickerton and Szathmáry, 2011. Before two million years ago, cut marks lie above bite marks, indicating that hominins accessed these bones only after other animals had. After two million years ago, bite marks more frequently lie above cut marks, indicating that hominins had first access to bones (Blumenschine, 1987; Blumenschine et al., 1994; Domínguez-Rodrigo et al., 2005; Monahan, 1996).
came from meat: Ben-Dor et al., 2021.
was an apex predator: Ibid.
almost absurd 85 percent meat: Ibid.
began to go extinct: From 1.8 to 1.5 million years ago had the highest per capita carnivoran extinction event (Bobe et al., 2007; M. E. Lewis and Werdelin, 2007; Ruddiman, 2008).
three times as fast: Perkins, 2013.
must have invented cooking: Wrangham, 2017.
time and energy digesting: Carmody and Wrangham, 2009.
50 percent become temporarily infertile: Koebnick et al., 1999.
ash in ancient caves: For evidence of fire use between 1.5 to 2 million years ago, see Gowlett and Wrangham, 2013; Hlubik et al., 2017; James et al., 1989.
after they are born: Garwicz et al., 2009.
in today’s human societies: This is inferred based on the shift in Homo erectus toward less sexual dimorphism (difference in body size between males and females); see Plavcan, 2012.
hunter-gatherer societies: Original grandmother hypothesis proposed by Hawkes et al., 1998. For another review with more detail on hunter-gatherer societies, see Hawkes et al., 2018.
full brain size achieved: Malkova et al., 2006.
origin of human languages: Christiansen and Kirby, 2010.
“own and my child”: Story from Terrace, 2019.
“problem in all of science”: Christiansen and Kirby, 2010.
language-ready vocal cords: D’Anastasio et al., 2013.
the survival of the species: D. S. Wilson and Wilson, 2007, provide a modern interpretation of group selection. The key idea is that evolution operates under multilevel selection, so group effects and individual effects are always interacting. In other words, the simple survival of the species (group-only effect) is not how evolution works. Traits that hurt an individual’s fitness but benefit the group overall are not necessarily selected for. Only in the right circumstances, where the individual cost is outweighed by the group benefit and competition with other groups.
Note that even these multilevel selection accounts of human evolution still acknowledge that defectors and cheaters were a problem that evolution would have had to account for. Thus, these multilevel accounts are still consistent with the evolutionary accounts in which mechanisms for the detection and punishment of violators would have been essential for stabilizing altruism and cooperation.
Once human groups had language, altruism, and the punishment of violators, the balance might have shifted toward strong group-selection effects, since altruism makes an individual’s fitness differences within a group muted (as members support and help each other), thereby strengthening the effect of across group competition.
where it is safest: W. D. Hamilton, 1971.
are around family members: R. Seyfarth and Cheney, 1990; Sherman, 1977, 1985.
called reciprocal altruism: Trivers, 1971.
them in the past: Mitani, 2006; Mitani and Watts, 1999, 2001; Povinelli and Povinelli, 2001.
that did not help them: Olendorf et al., 2004.
and language-enabled teaching: Mesoudi and Whiten, 2008.
among non-kin became possible: This story and ordering described here is largely that proposed by Fitch, 2010.
for defectors and liars: For arguments that lying, defectors, and cheating was an important obstacle to overcome in the evolution of language see Dunbar, 2004; Fitch, 2010; Knight, 2008; Tomasello, 2016. Dor, 2017, provides a more nuanced take, suggesting that the challenge of cheating (i.e., lying) that emerged with language may have driven more than one type of evolutionary feedback loop, not only for the punishment of violators after their discovery but also the ability to detect when someone was lying. As one individual gets better at lying, instead of making language unstable (selecting for worse language skills), it may have created selection for better theory of mind to better identify when people were lying and engaging with malintent. And this, in turn, created more pressure for liars to further hide their intent through better emotional regulation, which then created pressure for better theory of mind to see through the tricks, thereby creating a feedback loop.
human conversation is gossip: Dunbar et al., 1997.
The origins of language: Dunbar, 1998; Dunbar, 2004.
large group of individuals: For work on the importance of punishment in supporting altruism and its importance in the evolution of humans, see Boyd et al., 2003.
(hence no altruism required): Bickerton and Szathmáry, 2011.
language to evolve: Tomasello, 2016, 2018; as summarized in Dor, 2017.
trick for inner thinking: Berwick and Chomsky, 2017.
the last one million years: Morwood et al., 1999.
chimpanzee’s, perhaps even smaller: Falk et al., 2007.
tool use as Homo erectus: Sutikna et al., 2016.
Chapter 22: ChatGPT and the Window into the Mind
“fear of being turned off”: N. Tiku, 2022.
“nothing to fear from AI”: GPT-3, 2020. Note that the authors also did some editing of the article.
“they would fall over”: K. Lacker, (2020). Giving GPT-3 a Turing Test. Kevin Lacker’s blog. https://lacker.io/ai/2020/07/06/giving-gpt-3-a-turing-test.html.
four questions to GPT-3: This was in the GPT-3 sandbox, on text-davinci-002, test performed on Tuesday, June 28, 2022.
this the representative heuristic: Kahneman, 2013.
and produce meaningful speech: For TPJ in theory of mind, see Samson et al., 2004; Gallagher et al., 2000.
as false-belief tests: Cutting and Dunn, 1999; Hughes and Dunn, 1997; Jenkins and Astington, 1996. Note that the causation between mentalizing and language is controversial and unsettled. Astington and Jenkins, 1999, performed a longitudinal study that suggested that language skills predicted later performance on mentalizing tasks but not the reverse. But even if language dramatically improves mentalizing abilities, the basic ability to understand that other people have thoughts and agency seems to be a necessary foundation for joint attention to begin the process of naming objects in the first place (this is discussed in de Villiers, 2007).
similar impairments in language: Baron-Cohen, 1995.
“models of the underlying reality”: Posted on LinkedIn by Yann LeCun in January 2023.
Index
A specific form of pagination for this digital edition has been developed to match the print edition from which the index was created. If the application you are reading this on supports this feature, the page references noted in this index should align. At this time, however, not all digital devices support this functionality. Therefore, we encourage you to please use your device’s search capabilities to locate a specific entry.
NOTE: Italic page references indicate figures.
Abbeel, Pieter, 279–80
abiogenesis, 19–22
accumulation, 305–6, 307, 307–8, 308
acquisition, 82, 83, 84, 370
action potentials, 33, 33–34, 37, 38
active inference, 216–17, 223
active learning and AI, 278–80
actor-critic reinforcement learning, 106–7, 107, 118, 120, 121
acute stress response, 69–72
adaptation, 35–37, 36, 54, 74, 370
adrenaline, 70–72, 71
Adrian, Edgar, 32–37, 33
aerobic respiration, 23, 24, 374n
affect (affective states), 60, 60–63, 90, 370
of nematodes, 61–64, 62, 63
role of neuromodulators in first bilaterians, 65–67, 66
stress and worms, 69–72
African origins of modern humans, 238–39, 290, 324–28, 341, 343
agranular prefrontal cortex (aPFC), 206, 207, 208–9, 211–13, 216–20, 222–23, 223, 224, 226–30, 232, 255–60, 259, 370
AI. See artificial intelligence
akinetic mutism, 204–5, 206–7
“alien limb syndrome,” 229
all-or-nothing law, 33, 38
allyships, 249–50, 266
AlphaZero, 201–4, 211, 318
altruism, 333–36, 337–40, 339, 358
ALVINN (Autonomous Land Vehicle in a Neural Network), 278–79
American Sign Language, 299
amino acids, 18
amniotes, 159–60, 165n, 241
amphibians, 133, 159, 237
amygdala, 149, 150, 165, 166, 208, 219–20, 258, 286, 314–15, 321
anaerobic respiration, 23, 24
anemones, 29, 30, 38, 74, 80, 93
anhedonia, 73–74
Anomalocaris, 122–23
antelopes, 241–42, 302, 303, 328
anticipating future needs, 285–88, 289–90, 290, 295, 296, 360
Suddendorf and Bischof-Kohler hypothesis, 284–88, 392n
theory of mind and, 286–87, 287
antidepressants, 65
antioxidants, 21
antipsychotics, 65
ants, 94, 147–48
anxiety, 59, 65, 69–70
aphasia, 314–15
Aquinas, Thomas, 86
archosaurs, 161, 163
Aristotle, 13–14, 14, 295
arousal, 59, 60, 60–61, 73–75
arthropods, 93–94, 114n, 157–58, 377n
artificial intelligence (AI), 2–5, 11–12, 363–64
active teaching and, 278–80
brain and, 9–10, 11
challenge of pattern recognition, 127–28
continual learning problem, 81–82
first robot, 49–52
Minsky’s SNARC, 103–5
Mountcastle’s theory, 171
origins of term, 103
paper-clip problem, 352–53
theory of mind and, 265–66
artificial neural networks, 127, 127–28
artificial superintelligence (ASI), 265, 352, 363–64
associative learning, 78–81, 87–88, 90, 370
acquisition, 82, 83, 84, 370
blocking, 85, 85–86, 90, 104, 195, 370
continual learning problem, 81–84, 83
credit assignment problem, 84–86, 85
extinction, 82, 83, 371
overshadowing, 85, 85–86, 90, 104, 195, 371
reacquisition, 82–84, 83, 86, 90, 371
spontaneous recovery, 82–84, 83, 86, 90, 371
time course of, 82–84, 83
attention, 218–20, 318–20, 321, 336, 350
audition, 171, 172, 174
auditory cortex, 167–68, 170
Australopithecus, 323, 341
auto-association, 130–31, 135, 139, 151, 152, 176, 370
automation, 228, 229–30
avoidance, 52–53, 63, 79–80, 115–17, 219
axons, 32, 37, 130
baboons, 43, 243, 248, 283
backpropagation, 128, 137n, 139, 370
bacteria, 18–20, 48–49
Bagnell, Drew, 279
Barto, Andrew, 105–6
basal ganglia, 95–96, 96, 117–21, 152, 165, 208, 212–11, 215–16, 219–20, 229–30, 253–54
bees, 43, 94, 116, 116n, 147, 296
behavioral AI, 49–51
behavioral economics, 215
behavioral inhibition, 219–20
behavioral states, 62–63
Bentham, Jeremy, 43
Berridge, Kent, 67–68, 72
bilateral symmetry, 43, 44, 45–46, 370
bilaterians, xiv, 43, 44, 45, 45–56, 90, 370
affect, 74–75
associative learning, 80–81, 84–88, 132, 152, 302
chronic stress response, 72–75
credit assignment in, 195–96, 196
dopamine and, 114
early brain, 58, 80, 96, 153, 184
how they recognized things, 124–25, 125
prediction in, 184, 185
role of neuromodulators in affective states in, 65–67, 66
Roomba, 51–52
steering, 46–49, 49, 52–53, 81
synapses, 87–88
valence and, 52–55, 54, 57–58, 119
bipedalism, 325–26, 329, 395n
bird feathers, 340
birds, xiv, 13, 160n, 163, 164, 182, 196, 198, 238, 268, 317, 322, 335, 340
Bischof, Doris, 284
Bischof, Norbert, 284
Bischof-Kohler hypothesis, 284–88
blindness, 167, 170–71, 181, 183, 204
blocking, 85, 85–86, 90, 104, 195, 370
Boesch, Christophe, 276
bonobos, 244, 284, 297, 299–300, 313, 316, 364
bootstrapping, 107–9, 152, 259, 265, 361
Bostrom, Nick, 352
brain
AI and, 9–10, 11
anatomy, xiii, 5–6, 7, 95–96, 96, 253–54. See also specific regions
evolution. See brain evolution
first model of the world, 146–51
five breakthroughs, 10–11. See also specific breakthroughs
language in, 310–17, 338–40, 339
MacLean’s triune brain hypothesis, 8–9, 9
similarities across animal kingdom, 6–8
size of. See brain size
brain evolution, 6–8, 13–14, 93, 323, 323–24, 359–61
first mammals, 164–66, 166
five breakthroughs, 10–11, 39
social-brain hypothesis, 239–41
valence and nematodes, 52–55, 54
vertebrate template, 94–96, 95, 97
brain scaling, 253–55, 296
brain size, 239, 253–55, 254, 323, 323–24, 330
neocortex ratio, 240, 240–41
brainstem, 117–18, 312, 315
breakthroughs, 10–11, 359–65
#1: steering. See steering
#2: reinforcing. See reinforcement learning
#3: simulating. See simulation
#4: mentalizing. See mentalizing
#5: speaking. See language
evolution of progressively more complex sources of learning, 302, 302–3
Broca, Paul, 310–11
Broca’s area, 310–12, 311, 313–14, 316, 320–21
Brooks, Rodney, 49–51
Brunet-Gouet, Eric, 260
Buddhism, 192
buffalo, 241
Caenorhabditis elegans (C. elegans), 47, 47, 58, 375n
caloric surplus, 328–29, 358
Cambrian explosion, 93–94, 95, 140
Cambrian period, 93, 122–23
can’t-unsee property of perception, 174–75, 175
carbon dioxide, 20–22, 22, 57, 158
Carboniferous period, 159, 162
Carnegie Mellon University, 278, 279
catastrophic forgetting, 131–33, 135, 140, 199, 371
cats, 43, 186
learning, 97–101, 115
motor cortex, 223, 224, 224–25, 226
visual cortex, 135–36
causation vs. correlation, 195–96
cellular respiration, 21–23, 22
cephalopods, 157
Charles Bonnet syndrome, 181
ChatGPT, 2–3, 132, 344
chauvinism, 13
cheating, 333–34, 337–38, 396n
chess, 2, 105, 109, 200, 201
chimpanzees, xiv, 239
brain and brain size, 6, 240, 254, 290, 330, 342, 393n
communication, 296, 297, 299–300, 313, 315, 315–16, 319
diet and nesting locations, 282–84
grooming, 247, 249–50, 335
mating styles, 329
mental maps of, 244–46
motor cortex, 222
observational learning, 306
reciprocal altruism, 335
skill transmission, 273–77, 279
social structures, 243, 244–47, 250
theory of mind, 264
tool use, 267–68, 273
Chomsky, Noam, 340
chronic stress response, 72–75
classical conditioning, 76–79, 80, 82, 85–86
climate change, 158
Cnidarians, 379n
Coates, Adam, 279–80
“cocktail-party effect,” 174
Cohen, Neal, 131–32, 135
coincidence detection, 88n
communication, 296–99. See also language
altruism problem, 340
attempts to teach apes language, 299–301
emotional expressions, 314, 314–17
transferring thoughts, 301–7
concepts, 61, 301–2
conditional reflexes, 77–78
connectionism, 97–100
consciousness, 309, 390n
constrained hallucinations, 181–82
content-addressable memory, 130–31
continual learning problem, 81–84, 83, 371
catastrophic forgetting, 131–33
convolutional neural networks (CNNs), 137–40, 137n, 138, 139n, 371
cooking, 328–29, 358
cooperation strategies, 303–5, 304
copper barrier, 55, 56n, 57, 374n
corals (coral polyps), 29–31, 30, 31, 38, 47, 81, 90
correlation vs. causation, 195–96
cortex, 95–96, 96, 117, 129, 129–31, 133, 152
cortical columns, 168–72, 169, 211, 216–17, 386n
microcircuitry of, 171–72, 172
counterfactual learning, 192–96, 193, 232
cravings, 68, 219–20, 227–30
credit assignment problem, 84–86, 90, 104, 371
evolution of, 195–96, 196
original four tricks for tackling, 84–86, 85
temporal, 105–7, 113, 120, 152, 200, 371
Cretaceous period, 162
crows, 186, 267–68
cruelty, 12, 336, 340, 358
cultural bias and emotions, 59–60
curiosity, 142–45, 152, 382n
cyanobacteria, 19–21, 20, 24, 158, 238
cynodonts, 161, 162
Dale, Henry, 37
Damasio, Antonio, 204–5, 206, 217
dard, 59
Darwin, Charles, 7, 295, 330
Dawkins, Richard, 305
Dayan, Peter, 110, 112, 113, 175–77
DeCasien, Alex, 283–84
deception, 245, 252
declarative labels, 297–98, 300
decorrelation, 130
Deep Blue, 108–9
DeepMind, 142, 201
AlphaZero, 201–4, 211
deliberative choices, 208–13, 210
step #1: triggering simulation, 210, 210–11
step #2: simulating options, 210, 211–12
step #3: choosing an option, 210, 212–13
DeLong, Caroline, 139
Democritus, 86
dendrites, 32, 129
depression, 59, 65, 69, 73–74
Descartes, René, 86, 87
detour tasks, 190–92
Devonian period, 157–58, 162
de Waal, Frans, 239–40
diabetes, 378–79n
Dickinson, Tony, 213–14
diet, 238–39, 251–52, 282–84, 326, 327, 328–29
digestion, 28–29, 76–77
dinosaurs, 159–60, 160n, 161, 162, 163, 164, 233, 237–38, 241
disappointment, 115–17
discounting, 113
discrimination problem, 125–26, 126, 129–30, 130
dishwashing robots, 2, 4, 230
diurnal, 238
DNA, 18, 20, 304–5, 363
Dobzhansky, Theodosius, 7
dogs, xiv, 77–78, 82, 97, 186, 239, 242, 246–47, 274
dolphins, xiv, 238, 239, 239, 246, 274, 365
dominance, 242–43, 244, 247–48
dopamine, 64–69, 66, 88, 118, 119, 152, 165, 359, 376n, 381n
dorsal cortex, 165n, 383n
dreams (dreaming), 182, 183
drug addiction, 110, 144, 227–30
dualism, 86–87
Dunbar, Robin, 239–41, 282, 290, 337–38
East Side apes, 325, 325–26
Eccles, John, 37–38
ecological-brain hypothesis, 282–84, 290
Ediacaran period, 46, 46–48, 84, 93–94, 94
Edison, Thomas, 305
electricity, 4, 32, 305
electrophysiology, 32–33
elephants, xiv, 223, 238, 239, 267–68, 326
eligibility traces, 84–86, 85, 88, 90
Elman, Jeffrey, 317–18
“embodiment,” 224
emotion, categories of, 59–60
emotion, origin of, 59–75
the blahs and blues, 72–75
dopamine and serotonin, 64–69, 66
steering in the dark, 61–64
stress and worms, 69–72
emotional expression system, 315, 315–16, 394n
empathizing, 262
endurance running, 328
entropy, 17–18, 20, 363
Epicurus, 86
episodic memory, 13, 196–99, 232–33, 303, 385n
ether, 32
eukaryotes, 23–24, 24, 25, 28, 374n
euphoria, 68, 74
evagination, 383n
evolution, 359–62
arms race for political savvy, 237–39, 251–52
of the brain. See brain evolution
Cambrian explosion, 93–96
fungi, 27–31, 31
Homo Erectus and emergency of human hive mind, 336–41
Homo Erectus and rise of humans, 326–30
human lineage and proliferation, 13–15, 14, 323, 323–24, 341, 341–43
of language, 302, 302–3, 330–33, 332, 358–59
of nervous system, 26–27
neural dark ages, 157–66
origins of life, 17–22
Pavlov and origin of learning, 76–79
of prediction, 184, 185
of progressively more complex sources of learning, 302, 302–3
shared developmental stages for all animals, 28–29, 29
of temporal difference learning, 103–21
tension between the collective and the individual, 241–44
tree of life. See tree of life
exaptation, 340
excitatory neurons, 38, 65
executive control, 218
expansion recoding, 129–30, 130
exploitation, 66, 68, 376n
exploitation-exploration dilemma, 142–43, 152
extinction, 82, 83, 371
extinction events, 158–59
Late Devonian Extinction, 158–59, 162, 238
Permian-Triassic extinction event, 160–61, 237–38, 251
eye, 117, 135–37, 332–33
eyewitness testimonies, 197–98
Facebook, 144
facial expressions, 314, 314–15, 394n
dopamine and reward, 67, 67–68
Fadiga, Luciano, 268–69
false-belief tests, 261–62, 354, 389n, 397n
Sally-Ann test, 260–62, 261, 262, 264
fear, 61, 63, 117, 123, 125–26
female hierarchies, 248–49
ferrets, 170
Feynman, Richard, 10
field dependence, 229
fight-or-flight response, 70
filling-in property of perception, 173, 173
fire, use of, 328–29
firing rate, 33, 33–36, 371
first model of the world, 146–51
inner compass, 148–49
maps of fish, 146–48
medial cortex, 149–51
first move, 163–64
fish, 100–102, 193, 233, 334
avoidance tasks, 115, 116, 116n, 117
brain, 132–33, 139–40, 164–65, 165n
catastrophic forgetting, 132–33
communication, 296
evolution and tree of life, xiv, 157, 158–59, 162, 164–65, 194, 237, 241
invariable problem, 139–40
maps of, 146–48, 190–91, 384n
observational learning, 274–75, 275
reinforcement learning, 100–102, 110, 115, 144
smell and nose, 123–24, 124, 125–26
vestibular sense, 148, 148–49
flatworms, 49, 85, 116, 125
Fogassi, Leonardo, 268–69
forebrain, 95–96, 96, 119
Franklin, Benjamin, 4
freeloaders, 333, 335, 337
free time, 251–52
friendships, 250, 252
Friston, Karl, 216–17, 223–24
frugivores, 251–52, 282–84, 288
Fukushima, Kunihiko, 136–38
full signals, 58
fungi, 24, 27–31, 31, 31n
Gallese, Bittorio, 268–69
gambling, 144–45
gap junctions, 37, 37
gastrulation, 28–29, 29
generalization problem, 126, 126
generative mode (generative models), 177–81, 371
Helmholtz machine, 177–79, 178
neocortex as, 181–83, 188, 222, 258–60
predicting everything, 183–87, 185
StyleGAN2, 179, 179–81
genes, 18, 20, 304–5, 363
genome, 317
gestures, 296–97, 301, 310, 313–14, 315–16
Go (game), 2, 201–3, 202
goal-driven behavior, 213–17
goal hierarchy, 226–31, 228
Goodall, Jane, 267–68, 315–16
Google, 344
DeepMind, 142, 201
gorillas, 239, 243, 299–300, 313
gossip, 337–38, 339, 358
GPT-3 (Generative Pre-trained Transformer 3), 3–4, 344–51, 354–55, 355
GPT-4 (Generative Pre-trained Transformer 4), 354–56, 355
grammar, 297–98, 300, 336
“grandmothering,” 329
granular prefrontal cortex (gPFC), 206, 226, 255–60, 259, 262, 263, 289, 290, 371
granule cells, 206
Great Ape Dictionary, 296
Great Oxygenation Event, 21, 238, 374n
Great Rift Valley, 324–25
grief, 59–60
grocery shopping, 284–88
grooming, 247, 249–50, 335
group living, 241–44
group selection, 333–36, 337, 395n
habitual behavior, 213–15
Haldane, J. B. S., 334
hallucinations, 181–83
Harari, Yuval, 303
harems, 242–44, 243, 388n
Harvard University, 97
head-direction neurons, 149
Heath, Robert, 68
Hebb, Donald, 88
Hebbian learning, 88–89, 130
Helmholtz, Hermann von, 175–76, 180–82, 185
Helmholtz machine, 177–79, 178, 180, 182, 371
heroin addiction, 230
hindbrain, 95–96, 96, 149, 165
Hinton, Geoffrey, 6, 127–28, 175–77, 182
hippocampus, 149–51, 165, 190, 196, 198–99
Hippocrates, 31–32
Hobbes, Thomas, 86, 330
Homo erectus, 323, 326–30, 331–32, 341
emergence of the human hive mind, 336–41
Homo floresiensis, 341, 341–42
Homo neanderthalensis, 323, 331, 341, 342–43
Homo sapiens, 297, 301, 323, 331–32, 341, 342–43, 361
horses, xiv, 223, 238, 239
Hubel, David, 135–36, 137
human proliferation, 341, 341–43
human uniqueness, 295–309
attempts to teach apes language, 299–301
communication, 296–99
the singularity, 307–9
transferring thoughts, 301–7
Humphrey, Nicholas, 239–40
hunger, 58, 62, 79, 79–80, 119, 286, 287
hypothalamus, 95–96, 96, 119–21
IBM Research, 108–9
ideas, 301–2, 305–6, 307–8
illusions, 172
imagination, 182–83, 186–87, 303
imitation (imitation learning), 98–99, 274–75, 277–81, 289–90, 290, 306–7
AI and, 278–81
imperative labels, 297, 300
inductive bias, 138, 140
inference, 175–77, 180–82, 185
inhibitory neurons, 38, 65
inner compass, 148–49
inner ear, 124, 135, 140, 148–49
Instagram, 144
intention, 205, 208–9, 245–47, 257, 260
internal models, 146, 147, 151. See also models
intuitions, 60–61, 146
invariance problem, 133–40, 134, 151
inverse reinforcement learning, 277–81
invertebrates, 94–95, 95, 114n, 116, 144, 151, 157, 237
involuntary associations, 78
iPhone, 127
iRobot, 51
jellyfish, xiv, 27, 28, 29, 34, 38, 39, 43, 74, 80
Jennings, Ken, 109
Jetsons, The (TV show), 1–2, 132
Johns Hopkins University, 131, 135
Johnson, Adam, 190
joint attention, 318–20, 321, 336, 337, 358
Jurassic period, 162, 233
Kahneman, Daniel, 215
Kanada, 86
Kandel, Eric, 76
kangaroos, xiv, 223
Kanzi (bonobo), 299–300, 320
Kasparov, Garry, 108–9
kin selection, 334–36, 337
knowledge, 132, 246–47, 257
koalas, xiv, 223
lamprey fish, 95, 118–19, 123, 129
language, 185–86, 297–99, 309, 318–19
attempts to teach apes, 299–301
in the brain, 310–17, 338–40, 339
breakthrough #5 summary, 358, 360–61
emergence of the human hive mind, 336–41
evolution of, 302, 302–3, 330–33, 332, 358–59, 360
relationship between mentalizing and, 353–54
transferring thoughts, 301–7
language curriculum, 317–21
large language models (LLMs), 2–3, 344–50, 356–57
GPT-3, 3–4, 344–51, 354–55, 355
GPT-4, 354–56, 355
last universal common ancestor (LUCA), 19–20, 24
Late Devonian extinction, 158–59, 162, 238
latent inhibition, 85, 85–86, 90, 104, 195, 380n
Late Permian extinction event, 160–61, 237–38, 251
lateral cortex, 149–51, 150, 165, 166
law of effect, 99–100, 103, 144, 189, 213
layer four, 172, 206, 206n, 216, 217
Leakey, Louis, 267
Leborgne, Louis Victor, 310
LeCun, Yann, 10, 137n, 186, 200, 356
Lemoine, Blake, 344
limbic system, 8–9, 9
lizards, 159–60, 161
logic, 50, 185–86
luminance, 34–35, 35n
lying (liars), 334, 337, 396n
macaque monkeys, 222, 240, 243, 244, 256, 268, 313, 329, 330
McCloskey, Michael, 131–32, 135
Machiavellian apes, 244–47
machine learning, 12, 84
MacLean, Paul, 8–9, 371n
mammals. See also specific mammals
brain, 95, 113–14, 135–36, 149–50, 163–66, 166, 186–87, 203–4, 205, 205–7, 232–33, 253–55
control and, 218–20
credit assignment in, 195–96, 196
Era of Mammals, 238–39, 239
evolution and tree of life, xiv, 162, 163, 238–39, 239
evolutionary tension between the collective and the individual, 241–44
goals and habits, 213–15
inner duality of, 213–15
making choices, 209–13
motor cortex, 223, 223–26
motor hierarchy, 226–28, 227, 228
neocortex, 206–8, 207, 209, 209, 232–33, 256
neocortex ratio, 240, 240–41
prediction in, 184, 185
primate politics, 247–52
simulating actions, 163–64
visual cortex, 135–38
materialism, 86–87
medial cortex. See hippocampus
memes, 305
memory, 76, 116
attention and self-control, 218–20
catastrophic forgetting, 131–33
episodic, 196–99, 232–33
working, 187, 218, 219–20
mentalizing, 289–91, 290, 361, 371
breakthrough #4 summary, 289–91, 360
evolution of progressively more complex sources of learning, 302, 302–3, 360
relationship between language and, 353–54
Menzel, Emil, 244–45
Mestral, George de, 4
metacognition, 258
mice, 163–64, 226, 283, 296
midbrain, 95–96, 96, 110, 117, 165
mind. See models; theory of mind
Minsky, Marvin, 2, 103–5, 120, 200
mirror neurons, 268–73
mirror self-recognition tests, 257, 264
mirror-sign syndrome, 257–58
models (modeling)
first. See first model of the world
frontal vs. sensory neocortex in first mammals, 209, 209
mind to model other minds, 263–65
other minds, 260–63, 261
own mind, 258–60, 259
model-based reinforcement learning, 199, 199–200, 201–20, 371
AlphaZero, 201–4, 211, 318
attention, working memory, and self-control, 218–20
evolution of first goal, 215–17
goals and habits, 213–15
mammals making choices, 209–13
predicting oneself, 208–9
prefrontal cortex and controlling the inner simulation, 204–8, 205, 207
model-free reinforcement learning, 199, 199–200, 201, 211, 212, 215–16, 318, 359–60, 371
Molaison, Henry, 196–97, 198
mongooses, 267–68, 274, 275
monkeys, xiv, 194, 247–48, 269–71, 284–85, 287–88, 316
Montague, Read, 110, 112, 113
Morse code, 33
motivation, 73–74
motor cortex, 206, 221–26, 222, 232, 241, 360
language and, 312
leading theory on evolution of, 222–23, 223
mirror neurons, 268–73
missing layer four, 206, 206n
predictions, 223–26
motor hierarchy, 226–31, 227, 228
motor planning, 224–26, 270, 271
Mountcastle, Vernon, 168–70, 289
multicellular organisms, 24, 24–26, 25, 28
multi-male groups, 242–44, 243, 387n
myths, 303–4, 304
Naqshbandi, Miriam, 284–85, 285n, 287–88
natural selection, 330, 340, 363
nature and intelligence, 4–6
‘nduh, 59
negative-valence neurons, 53–55, 54, 56–57, 61, 100
nematodes, xiv, 46–48, 47, 94, 101, 147
affective states of, 61–64, 62, 63
dopamine and serotonin, 64–69, 66, 114
problem of trade-offs, 55–57, 56
steering, 46–49, 48, 49, 53–54, 54
stress, 69–71, 73–74
temporal difference learning, 115–16, 116n
tweaking goodness and badness of things, 79, 79–80
valence and, 52–55, 54
neocortex, 8–9, 9
anatomy, 167–72, 168, 205. See also agranular prefrontal cortex; cortical columns; granular prefrontal cortex; motor cortex
counterfactual learning, 192–96, 193
episodic memory, 196–99
evolution, 163–64, 165–66, 166, 188, 289–90
functions, 218–20, 289–90
as a generative model, 181–83, 188, 222, 258–60
language and, 312–17, 315
layers, 169, 171–72, 172
MacLean’s triune brain hypothesis, 8–9, 9
new neocortical regions of early primates, 255–56, 256
new regions in primates, 255–56, 256, 263–64
perception, 172–75
prediction, 183–87, 185
ratio, 240, 240–41
sensory. See sensory neocortex
use of term, 167n
vicarious trial and error, 189–92
neocortical columns. See cortical columns
nepotism, 252
nerves, 32
nervous system, 26–27, 32
nervus, 32
neural circuits, 38–39, 39, 56, 86, 90
Neurogammon, 109
neuromodulators, 64–69, 66, 70–72, 71, 88, 165, 359, 371. See also specific neuromodulators
role in affective states of first bilaterians, 65–67, 66
neurons, 5, 7, 19, 26, 26–27, 28–29, 31–32
Adrian’s discoveries, 32–37, 33
cortical column, 168–72, 169
history of neuroscience, 31–39
negative-valence, 53–55, 54, 56–57, 61
positive-valence, 53, 54, 56–57
response of dopamine to predictive cues, rewards, and omissions, 110–14, 112
neurotransmitters, 37–38, 87
Newton, Isaac, 32
New York University (NYU), 283–84
Ng, Andrew, 279–80
NMDA receptors, 88n
Nobel, Alfred, 76
Nobel Prize, 32, 37, 76
nocturnal, 238
nonassociative learning, 80n
norepinephrine, 70, 123, 377–78n
observational learning, 272–77, 275, 280–81, 306, 360
“obstetric dilemma,” 329
octopamine, 70, 377n
octopuses, xiv, 14, 15, 157, 267–68, 275, 364
Oldowan tools, 326–27, 327
olfactory neurons, 123–30, 124, 129, 135
expansion and sparsity, 129–30, 130
olfactory receptors, 123–24, 124, 381n
one-at-a-time property of perception, 173–74, 174
On the Origin of Species (Darwin), 7, 330
OpenAI, 132, 354, 355, 356
opioids, 70–72, 71, 74
opposable thumbs, 238
origin of emotion. See emotion, origin of
origins of life, 17–22
orthogonalization, 130
overshadowing, 85, 85–86, 90, 104, 195, 371
oxygen, 21, 27
Oxygen Holocaust, 21
pair-bonding mammals, 242–44, 243, 329
paper-clip problem, 352–53
parasitic strategy, 28n
Parkinson’s disease, 118
pattern recognition, 122–41, 165
catastrophic forgetting, 131–33
computers and, 127–28
cortex, 129, 129–31
discrimination problem, 125–26, 126
generalization problem, 126, 126
invariance problem, 133–40, 134
problem of recognizing a smell, 123–26
pattern separation, 130, 133
Pavlov, Ivan, 76–79, 80, 82, 85–86, 98
Pellegrino, Giuseppe di, 268–69
perception, 172–75, 218
can’t-unsee property of, 174–75, 175
filling-in property of, 173, 173
one-at-a-time property of, 173–74, 174
Permian, 159, 160, 161, 162, 169
Permian-Triassic extinction event, 160–61, 237–38, 251
persistence hunting, 328
phagotrophy, 23–24, 28
photosynthesis, 20–22, 22, 23, 24, 27
physics, 17–18, 195–96, 350, 363
Pinker, Steven, 353
placoderms, 157
Plato, 86, 87, 330
political power, 247–52
Pomerleau, Dean, 278–79
positive-valence neurons, 53, 54, 56–57, 100, 119
predation, 93, 122–23, 243
predictions, 208–13, 210, 223–26
evolution of, 184, 184–85
motor commands and, 223–26, 271
neocortex and, 183–87, 209
reward-prediction, 111, 113, 114n, 115, 213–14
step #1: triggering simulation, 210, 210–11
step #2: simulating options, 210, 211–12
step #3: choosing an option, 210, 212–13
predictive cues, 84–86, 111, 112, 121
prefrontal cortex, 209. See also agranular prefrontal cortex; granular prefrontal cortex
controlling the inner simulation, 204–8, 205, 207
premotor cortex, 226, 229, 230
mirror neurons, 268–73
primates. See also specific primates
acquiring novel skills through observation, 275–77
anticipating future needs, 285–88
counterfactual learning, 194–95
ecological-brain hypothesis, 282–84, 290
evolution and tree of life, xiv, 238–39, 239, 243–44, 289–91
evolution of progressively more complex sources of learning, 302, 302–3
modeling mind to model other minds, 263–65
modeling other minds, 260–63, 261
modeling own mind, 256–60, 259
motor cortex, 206, 221, 222, 222–23, 223, 268–73
neocortex, 240, 240–41, 313–14, 360
new neocortical regions of, 255–56, 256, 263–64
skill transmission, 273–77, 275
social-brain hypothesis, 239–41, 282
social politics, 247–52, 281
social structures, 242–44, 243
theory of mind. See theory of mind
tool use, 267–68, 273–75
visual cortex, 253–55, 254
primate sensory cortex (PSC), 255, 258–59, 354, 371
procedural memory, 197
proteins, 18–19
protein synthesis, 18–19
proto-conversations, 318–20, 336–37
protolanguages, 331–32, 336, 358
psychedelics, 65
psychic stimulation, 77–78
punishment, 337–38, 358, 396n
puzzle boxes, 98, 98–99, 99, 101, 103, 115, 277, 306
radial symmetry (radiatans), 43, 44, 45, 53, 54, 80
Ramón y Cajal, Santiago, 37
rate coding, 34–37, 36, 38
rats, xiv
anticipating future needs, 284–85, 285n, 287
brain, 8, 78, 149, 150, 169, 189–90, 198–99, 206, 207, 213–14, 223, 224, 229
detour tasks, 191–92
dopamine and pleasure, 66
dopamine and stimulation, 65, 66–69, 110
episodic memory, 198–99
observational learning, 274, 276–77
regret in, 193, 193–94
role of habits, 213–14
role of play, 241
spinal cord, 78, 86
variable-ratio reinforcement, 144
vicarious trial and error, 189–90, 191–92, 209–10, 212, 220
reacquisition, 82–84, 83, 86, 90, 371
reciprocal altruism, 335–36
reciprocity, 250, 252
recognition. See also pattern recognition
mirror self-recognition tests, 257, 264
neocortex and, 182–83, 188
recognition modes, 177–79, 178
Redish, David, 190, 193, 193–94
register-addressable memory, 130–31
regrets, 192, 193, 193–94
reinforcement learning, 101–6, 164–65, 192–93, 359–61
based on actual rewards, 107–8, 108
based on temporal differences in expected rewards, 107–8, 108
breakthrough #2 summary, 152–53, 359–60
evolution of progressively more complex sources of learning, 302, 302–3
importance of curiosity in, 142–45
model-based. See model-based reinforcement learning
model-free. See model-free reinforcement learning
Thorndike and, 96–101
relief, 115–17
REM sleep, 182, 384n
reptile brain, 8–9, 9
reptiles, xiv, 159–61, 162, 165, 165n, 296
respiration, 21–23, 22, 27, 374n
ribosomes, 18
Rizzolatti, Giacomo, 268–69
Roberts, William, 284–85, 285n, 287–88
robotics
first robot, 49–52
imitation learning, 278–81
Rochester Institute of Technology, 139
rock, paper, scissors (game), 194–95
Roomba, 51, 51–52, 53, 58, 64
Rosey the Robot, 1–2, 5, 51, 132
Ross, Stephane, 279
Rousseau, Jean-Jacques, 330
Rumelhart, David, 127–28
rumination, 192–93
salamanders, 159
Salk Institute, 110
Sally-Ann test, 260–62, 261, 262, 264
salt, 79, 79–80, 81
Sapiens (Harari), 303
satiation, 62, 62, 63, 66, 69, 287
Savage-Rumbaugh, Sue, 300
“scale of nature,” 14
Schultz, Wolfram, 111–13, 112, 115
search problem, 200, 202, 203, 209, 211, 232
Searle, John, 303
second law of thermodynamics, 17–18
sehnsucht, 59–60
seizures, 196–97, 198
selective serotonin reuptake inhibitors (SSRIs), 378n
self-concept (sense of self), 217, 264, 390n
self-control, 219–20
self-driving cars, 278–79
Selfish Gene, The (Dawkins), 305
self-reference, 257
self-replication, 18, 19
semicircular canals, 148, 148–49
sensitization, 80n
sensory neocortex, 197, 198, 205, 205–6, 211–13, 216–17, 232, 258–59, 371
in first mammals, 209, 209
serotonin, 64–69, 66, 71–72, 73, 88, 359, 376n, 378n
Sherrington, Charles, 37
sign language, 299, 311–12
simulation, 163–64, 361
breakthrough #3 summary, 232–33, 360
evolution of progressively more complex sources of learning, 302, 302–3, 360
GPT-3 and LLMS, 349–51
hierarchy of goals, 228, 229–30
making choices and, 210, 210–13
survival by, 163–64
simulation theory, 263–64
skill transmission, 273–77, 275
Skinner, B. F., 100, 144
sleep, 181, 182
smell, 34, 38, 47, 53–54, 123–26, 135. See also olfactory receptors
Smith, Neil, 312
snakes, 159–60, 162
social-brain hypothesis, 239–41, 282, 290
social groups, 241–44
social hierarchy, 242–44, 247–52, 265–66
social media, 144–45
social projection theory, 263–64, 389n
solitary mammals, 242–44, 243
“source of error,” 77
spandrels, 340
spatial maps, 146–48
vestibular sense, 148, 148–49
speaking. See language
spiders, 93, 158, 364
spike (firing) rate, 33, 33–36, 371
spontaneous recovery, 82–84, 83, 86, 90, 371
squirrel monkeys, 284–85, 287–88
squirrels, 163, 226, 271
“squishing problem,” 35–37
steering, 46–49, 49, 57–58, 61–64, 64
bilaterians, 46–49, 49, 52–53
breakthrough #1 summary, 90, 359
Roomba, 51–52, 53, 64
“steer in the dark,” 64
Steiner, Adam, 193–94
stimulants, 65
stimulus strengths, 33–34, 34, 36
Stochastic Neural-Analog Reinforcement Calculator (SNARC), 103–5
stress, 69–72, 71, 90
acute stress response, 69–72
ancient stress cycle, 71–72, 72
chronic stress response, 72–75
stroke victims, 171, 204–5, 221, 222
StyleGAN2, 179, 179–81
submission, 242–43, 247–48
Suddendorf, Thomas, 284, 285, 286–88, 392n
sugar, 20, 21–22, 27–28
Superintelligence (Bostrom), 352
superior temporal sulcus (STS), 255n, 371
supervised learning, 128, 176, 180
Sutton, Richard, 105–9, 113, 118, 120, 121, 142–43, 203
sweat (sweating), 328
symbolic AI, 49–51
symbols, 297–98, 300
synapses, 37, 37–38, 87–89, 88, 118, 371
system 1 thinking, 215
system 2 thinking, 215
TD-Gammon, 109, 110, 142, 201, 318, 380n
temperature navigation, 54–55
temporal credit assignment problem, 105–7, 113, 120, 152, 200, 371
temporal difference learning (TD learning), 103–21, 106, 142–43, 152, 198–99, 203, 371
basal ganglia, 117–21
emergence of relief, disappointment, and timing, 115–17
exploitation-exploration dilemma, 142–43, 152
grand repurposing of dopamine, 110–14
magical bootstrapping, 105–9
temporal difference signals (TD signals), 107, 111–14, 152, 372
temporoparietal junction (TPJ), 255n, 256, 354, 372
terraforming of Earth, 19–22
Tesauro, Gerald, 108–9, 110
tetrapods, 159, 162
thalamus, 95–96, 96, 117, 133, 134, 139–40, 172, 172, 382n
theory of mind, 246–47, 260–66, 268, 289–90, 290, 372
acquiring novel skills through observation, 275–77
anticipating future needs and, 286–87, 287
childhood development and, 264, 390n
modeling mind to model other minds, 263–66
politicking and, 281
Sally-Ann test for, 260–62, 261, 262, 264
therapsids, 160–61, 162
Thinking, Fast and Slow (Kahneman), 215
thispersondoesnotexist.com, 179, 179–80
Thorndike, Edward, 96–100, 98, 101, 110, 111, 115, 189
Thorpe, Chuck, 278–79
thought transfer, 301–7
time perception, 173–74, 174
timing, 116–17, 152
Tolman, Edward, 189–90, 244
tool use, 267–68, 273–75, 284, 327–28, 358
Oldowan tools, 326–27, 327
trade-offs, 55–57, 56
translation, 139
transmissibility, 273–77, 275
tree of life, xiv, 23–25, 24, 43, 45, 162
Cambrian ancestors, 94–95, 95
humans, 341, 341–43
mammals, 238–39, 239
neuron-enabled animals, 29–30, 30
radial vs. bilateral symmetry, 44
trial-and-error learning, 99, 99–100, 101, 103–4, 110–11, 142–43, 152
vicarious, 189–92, 211, 212–13, 232–33, 360, 361
Triassic period, 162
Permian-Triassic extinction event, 160–61, 237–38, 251
tribalism, 252, 364
triune brain hypothesis, 8–9, 9, 373n
Tsimpli, Ianthi-Maria, 312
Turing, Alan, 103
turtles, 159–60, 162, 319
Tyrannosaurus, 161
uncertainty, 210–11, 214
unconditional reflexes, 78
unconscious inference, 175–77, 180–82, 185
ungating, 117–18, 120, 381n
University College London, 216
University of California, Berkeley, 189
University of California, San Diego, 317
University of Massachusetts Amherst, 105–6
University of Michigan, 67
University of Minnesota, 190
University of Parma, 268–69
University of Western Ontario, 284
unsupervised learning, 176
utilization behavior, 229
V1 (visual area 1), 135–37, 136
V2 (visual area 2), 136, 136
V4 (visual area 4), 136, 136
valence, 4, 52–59, 54, 90, 119, 372
variable-ratio reinforcement, 144
Velcro, 4
ventral cortex, 149–51, 150, 165, 166
Venus flytraps, 30n
vertebral column, 94
vertebrates. See also specific vertebrates
brain, 94–96, 97, 110–11, 118–19, 120, 120–21, 122, 129, 129, 132–33, 139–40, 140, 149, 153, 164–66, 259
cortex, 129, 129–31, 149–51, 151, 164–66, 166
credit assignment in, 195–96, 196
evolution and tree of life, xiv, 94–96, 95, 96, 100, 162, 233, 360
how they recognized things, 124–25, 125
prediction in, 184, 185
smell and nose of, 123–26, 124
temporal difference learning, 110, 114, 115–16, 118–19, 143–44, 192–93, 194
vervet monkeys, 247–48, 297, 301–2, 335
vestibular sense, 148, 148–49
vicarious trial and error, 189–92, 211, 212–13, 232–33, 360, 361
vision, 34–35, 124, 172–75
invariance problem, 133–40, 134
visual cortex, 134–37, 139, 167, 170, 253–55, 254
cortical column, 168–72, 169
volition, 216
“wake-sleep algorithm,” 182
Wallace, Alfred, 330–32
wanting, 68–69, 111, 114, 114n
warm-bloodedness, 160–61, 160n, 162, 163, 164–65
Washburn, Sherwood, 329
Wernicke, Carl, 311
Wernicke’s area, 311, 311–12, 313–14, 316, 320–21, 354
whales, xiv, 238, 239, 322
Wiesel, Torsten, 135–36, 137
wildebeests, 334
Williams, Ronald, 127–28
willpower, 219–20
working memory, 187, 218, 219–20
world models, 186, 200, 209, 232
Wrangham, Richard, 328
wrasses fish, 268
About the Author
MAX BENNETT is the cofounder and CEO of Alby, an AI company based in New York City. Max was previously the cofounder and chief product officer of Bluecore, a company that uses AI to help some of the largest brands in the world personalize their marketing. Bluecore was recently valued at over $1 billion, ranked numerous times on the Inc 500 fastest-growing companies in the US, and made the 2018 Glassdoor Best Places to Work list. Prior to Bluecore, Max was a trader at Goldman Sachs. Max holds several patents for AI-related technologies, and has published numerous research papers in peer reviewed journals on the topics of evolutionary neuroscience and intelligence. Max graduated from Washington University in St. Louis, summa cum laude, with a BA in economics and mathematics, where he won the John M. Olin prize for the best economics honors thesis. In 2016, Max was featured on the Forbes 30 under 30 list. Max lives in Brooklyn, New York, with his wife, Sydney, and their dog, Charlie.
Discover great authors, exclusive offers, and more at hc.com.
Praise for A Brief History of Intelligence
“This book discloses everything you always wanted to know about the brain (but were afraid to ask). It is an incredible resource. It assimilates every discovery in neuroscience—over the last century—within a beautifully crafted evolutionary narrative. The ensuing story shows how an incremental elaboration of brains can be traced from ancient worms to the mindful, curious creatures we have become. The synthesis works perfectly. Its coherence obscures the almost encyclopedic reach of this treatment.”
—Karl Friston, University College London, the #1 most-cited neuroscientist in the world
“Max Bennett published two scientific papers on brain evolution that blew me away. Now he has turned these into a fabulous book, A Brief History of Intelligence. His friendly writing style, clear jargon-free prose, and well of information make this book a winner.”
—Joseph LeDoux, New York University, bestselling author of Anxious and The Deep History of Ourselves
“With a truly mind-boggling scope, A Brief History of Intelligence integrates the most relevant scientific knowledge to paint the big picture of how the human mind emerged. . . . This text is embracing, ambitious, and lusciously enlightening but still remains strictly orientated to the facts, and avoids unsubstantiated speculation. This is both a piece of art as well as science. . . . I am deeply impressed by this brave project of explaining entire human nature in the grand evolutionary frame. But I am even more impressed that Max Bennett succeeded in this virtually impossible task.”
—Kurt Kotrschal, University of Vienna, winner of 2010 Austrian Scientist of the Year Award and author of the critically acclaimed Wolf-Dog-Human
“Written with gusto and spirit, with intellectual courage and playfulness. It is eye-opening and intellectually invigorating . . . the work of a young and fresh mind that has no axes to grind and comes to the subject with untarnished joyful curiosity, intelligence, and courage. Everyone, from young students to established academics will find it rewarding.”
—Eva Jablonka, Tel Aviv University, coauthor of Evolution in Four Dimensions and The Evolution of the Sensitive Soul
“Max Bennett gives a lively account of how brains evolved and how the brain works today. A Brief History of Intelligence is engaging, comprehensive, and brimming with novel insights.”
—Kent Berridge, professor of psychology and neuroscience at University of Michigan and winner of the Grawemeyer Award for Psychology
“If you’re in the least bit curious about that three-pound gray blob between your ears, read this book. Max Bennett’s entertaining and enlightening natural history of brains is a tour de force—as refreshing as it is entertaining. It made my brain happy.”
—Jonathan Balcombe, PhD, bestselling author of What a Fish Knows and Super Fly
“This book provides an exciting journey through the keys to human intelligence and has important things to say about who we are and what it means to be human. The five ‘breakthroughs’ in which the ability to interact with the world gets more and more complex provides a novel evolutionary structure that carries the story forward. Well written in a very readable and engaging style. Highly recommended.”
—A. David Redish, University of Minnesota, author of The Mind within the Brain and Changing How We Choose: The New Science of Morality
“If you are interested in understanding brains or in building human-like general AI, you should read this book. This is a forward-looking book masquerading as history. A mind-boggling amount of details of anatomy, physiology, and behavior of a variety of nervous systems are brought together in a coherent evolutionary tale and explained in their computational contexts. It is a joy to read—don’t miss it!”
—Dileep George, DeepMind, previously cofounder of Vicarious AI
Copyright
A BRIEF HISTORY OF INTELLIGENCE. Copyright © 2023 by Max Solomon Bennett. All rights reserved under International and Pan-American Copyright Conventions. By payment of the required fees, you have been granted the nonexclusive, nontransferable right to access and read the text of this e-book on-screen. No part of this text may be reproduced, transmitted, downloaded, decompiled, reverse-engineered, or stored in or introduced into any information storage and retrieval system, in any form or by any means, whether electronic or mechanical, now known or hereafter invented, without the express written permission of HarperCollins e-books.
FIRST EDITION
Cover design by Brian Moore
Cover images © Getty Images
Library of Congress Cataloging-in-Publication Data
Names: Bennett, Max S. (Max Solomon), author.
Title: A brief history of intelligence : evolution, AI, and the five breakthroughs that made our brains / Max S. Bennett.
Description: First edition. | New York : Mariner Books, [2023] | Includes bibliographical references and index.
Identifiers: LCCN 2023008730 | ISBN 9780063286344 (hardback) | ISBN 9780063286368 (ebk)
Subjects: MESH: Brain--physiology | Biological Evolution | Artificial Intelligence | Intelligence--physiology
Classification: LCC QP376 | NLM WL 300 | DDC 612.8/2--dc23/eng/20230727
LC record available at https://lccn.loc.gov/2023008730
Digital Edition OCTOBER 2023 ISBN: 978-0-06-328636-8
Version 09042023
Print ISBN: 978-0-06-328634-4
About the Publisher
Australia
HarperCollins Publishers Australia Pty. Ltd.
Level 13, 201 Elizabeth Street
Sydney, NSW 2000, Australia
Canada
HarperCollins Publishers Ltd
Bay Adelaide Centre, East Tower
22 Adelaide Street West, 41st Floor
Toronto, Ontario, M5H 4E3
India
HarperCollins India
A 75, Sector 57
Noida
Uttar Pradesh 201 301
New Zealand
HarperCollins Publishers New Zealand
Unit D1, 63 Apollo Drive
Rosedale 0632
Auckland, New Zealand
United Kingdom
HarperCollins Publishers Ltd.
1 London Bridge Street
London SE1 9GF, UK
United States
HarperCollins Publishers Inc.
195 Broadway
New York, NY 10007
* I asked GPT-3 to complete the following sentence: “I am in my windowless basement, and I look toward the sky, and I see . . .” GPT-3 said “a light, and I know it is a star, and I am happy.” In reality, if you looked upward in a basement you would not see stars, you would see the ceiling. Newer language models like GPT-4, released in 2023, successfully answer commonsense questions like this with greater accuracy. Stay tuned for chapter 22.
* Although systems don’t necessarily get more complex, the possibility of complexity increases over time.
* Except for the battle between bacteria and viruses, but that is a whole different story.
* Although, as with everything in evolution, there is nuance. There is a third, middle option that some species of both animals and fungi settled into: the parasitic strategy. Instead of actively catching prey to kill it, parasites infect prey and steal sugar or kill them from the inside.
* The Venus flytrap is a fascinating exception to this, an example of a plant that independently evolved the ability to capture prey by moving quickly.
* You could, of course, state this the opposite way: fungi never got to feed on other life because they never got neurons. The point is not which came first but that neurons and hunting level-two multicellular life were part of the same strategy, one which fungi never used.
* More specifically, the luminance is one million times larger. Luminance is measured in candelas per square meter, which is the rate that photons are generated per unit of surface area weighted by human wavelength sensitivity.
* Of course, this is mostly. There are cases when neurons touch each other and form gap junctions that allow the transfer of electrical signals directly from one neuron to another.
* In this experiment, researchers confirmed that this effect was not caused just by overexposure to salt, but instead by the association between a stimulus (salt) and the negative affective state of hunger. Researchers took a third group and made them spend the same number of hours in salt water but also added food to the dish so nematodes wouldn’t experience hunger. This third group, which experienced the same amount of exposure to salt, still happily steered toward salt afterward. This suggests the salt avoidance was not caused by overexposure to salt, but by the association between salt and hunger (see the middle example in figure 4.1).
* These more distant animals do engage in what is called nonassociative learning, such as adaptation (as Edgar Adrian found), and another similar type of learning called sensitization, which is when reflexes strengthen in response to a previously arousing stimulus.
* The protein machinery for this is beautiful but beyond the scope of this book. If interested, look up coincidence detection using NMDA receptors.
* Dopamine neurons always have a background static, firing at about one to two spikes per second. During these omissions, these neurons go silent (see figure 6.3).
* It is important to note that some invertebrates, specifically arthropods, do show such reward-prediction errors, but this is believed to have evolved independently given the fact that these reward-prediction errors are not found in other simple bilaterians, and the fact that, in arthropods, the brain structures these responses are found within are uniquely arthropod brain structures.
* In fact, recent studies show how elegantly evolution modified the function of dopamine while still retaining its earlier role of generating a state of wanting. The amount of dopamine in the input nuclei of the basal ganglia (called the “striatum”) seems to measure the discounted predicted future reward, triggering the state of wanting based on how good things are likely to be and driving animals to focus on and pursue nearby rewards. As an animal approaches a reward, dopamine ramps up, peaking at the moment when an animal expects the reward to be delivered. During this ramping-up process, if predicted rewards change (some omission or new cue changes the probability of getting a reward), then dopamine levels rapidly increase or decrease to account for the new level of predicted future reward. These rapid fluctuations in dopamine levels are produced through the bursting and pausing of dopamine neurons that Schultz found; these rapid fluctuations in dopamine levels are the temporal difference learning signal. The quantity of dopamine floating around in the striatum modifies the excitability of neurons, which shifts behavior toward exploitation and wanting. In contrast, the rapid changes in dopamine levels trigger modifications in the strength of various connections, thereby reinforcing and punishing behaviors. In other words, dopamine in vertebrates is both a signal for wanting and a signal for reinforcement.
* It requires some experimental cleverness to distinguish between an association merely fading because a contingency no longer applies (e.g., a light no longer leads to a zap) and learning from the omission of something. In one study with fish, the distinction was shown by adding a new cue specifically in trials where rewards were omitted. If an association were merely fading, then this new cue would not become rewarding (nothing was reinforced in the omission trial), but if instead an animal’s brain treats an omitted zap as rewarding in and of itself, then this new cue (which showed up uniquely when zaps were omitted) should be learned to be as rewarding. Researchers have shown that in such experiments fish do, in fact, treat this new cue as rewarding and will approach it in the future. In contrast, we know a nematode cannot do this because they cannot even associate events separated in time, and there is evidence (although it is still unsettled) that even smart invertebrates such as honeybees and crabs do not learn from omission in this way.
* There are 250 possible combinations of 50 elements that can be either on or off: 250 = ~1.1 x 1015
* Note that he did not use the word convolution, but he is credited with coming up with the approach and architecture. Also note that it was Yann LeCun who updated this architecture to use backpropagation, which is what catalyzed the widespread adoption of convolutional neural networks in practical applications.
* The way modern CNNs get around this rotation problem is by augmenting the training data to include huge numbers of examples of the same object rotated.
* Note that some dinosaurs, later in their evolutionary story, are believed to have evolved warm-bloodedness, as evidenced by chemical analyses of their fossils and the fact that birds are warm-blooded.
* Although, to be fair, there are differences between fish and reptile brains. Some argue amniotes evolved a dorsal cortex, a possible precursor to the neocortex (although newer evidence suggests the dorsal cortex was not present in early amniotes).
* Note that when referring to regions of neocortex in the brains of mammals, it is common to drop the neo—for instance, visual cortex rather than visual neocortex.
* If you don’t see this, look at the staircase and, while maintaining your gaze, rotate the page 180 degrees.
* Note that the motor cortex is also missing layer four, but it is not considered prefrontal cortex.
* Similar things may have independently occurred in other lineages of mammals with complex sociality (such as dogs, elephants, and dolphins).
* As mentioned in chapter 11, it is called granular because of its uniquely thick layer four, which contains granule neurons.
* The main new areas are the superior temporal sulcus (STS) and the temporoparietal junction (TPJ).
* ALVINN controlled the steering wheel only, not braking or acceleration.
* Note that Naqshbandi and Roberts did an initial baseline experiment to ensure the quantity of dates and the quantity of raisins each induced similar levels of thirst in monkeys and rats, as measured by the relative increase in water intake when animals were given free access to water alongside these quantities of dates or raisins.
* And incorporating other modalities directly into these models. Indeed, already newer large language models like GPT-4 are now being designed to be “multimodal,” whereby they are also trained in images in addition to text.
Settings
Theme
Ask about this book
Ask anything about this book.


